Table 1: Characteristics by of Multiple Sclerosis Cohort Grouped by Disease Subtype

Cox L, Cameron AP, Wittman D, Papin JE, Mao-Draayer Y, He C, Clemens JQ, Wei JT, Sarma AV, Stoffel JT*
Department of Urology, University of Michigan, 1500 E. Medical Center Dr. SPC 5330 Ann Arbor, MI, USA*Corresponding author: John Stoffel, MD, University of Michigan,Department of Urology 1500 E. Medical Center Dr. SPC 5330 Ann Arbor, MI, USA, Tel: 48109-5330; E-mail: jstoffel@med.umich.edu
Background: Although urinary symptoms are prevalent in multiple sclerosis (MS), there is little information whether bladder function differs by gender or disease subtype.
Objective: Differences in MS bladder function within subgroups were investigated by comparing female to male and relapsing remitting (RRMS) to secondary progressive (SPMS) patients.
Methods: We reviewed 118 MS patients referred for urologic evaluation between 2007 and 2012 and extracted demographic, questionnaire (AUASI, M-ISI), and urodynamic data. Variables were analyzed by gender and MS subtype, and a multivariable regression model was generated to adjust for age and gender
Results: The cohort consisted of83/35 female/male and 57/61RRMS/SPMS subjects. Urinary questionnaire and urodynamic findings were similar between genders, with the exception of higher maximum voiding pressures in males (p=0.003). RRMS patients reported more bothersome urinary symptoms compared to SPMS (AUASI21 vs. 15, p=0.004) and RRMS was independently associated with higher symptom scores on multivariable analysis (OR 17.1, p=0.008). There were no differences in urodynamic findings between subtypes.
Conclusions: Male and female MS patients had similar urinary symptom scores and urodynamic findings, with the exception of higher voiding pressures in males. RRMS patients reported significantly more severe urinary symptoms on AUASI, compared to SPMS, despite having similar urodynamic findings
Multiple sclerosis; Neurogenic bladder; Urodynamic testing; Gender
Multiple sclerosis (MS) is the most common neuroinflammatory disease of the central nervous system and affects approximately 85 per 100,000 people. The disease occurs in women two to four times more frequently than in men [1-3] and studies suggest that there are gender differences regarding the speed of disease progression, motor symptom severity, and cognitive function of people affected by MS [4,5]. The disease is categorized into several major subtypes based on clinical course and progression of neurologic symptoms: relapsing remitting (RRMS), secondary progressive (SPMS), and primary progressive (PPMS). The severity of neurologic symptoms can vary significantly between disease subtypes. RRMS patients have relapses (flare-ups) with various neurological symptoms separated by remitting periods. Over 50% of RRMS patients convert to SPMS after 10-15 years with the disease [6]. SPMS patients have gradual neurological deterioration (progression) with increasing physical debilitation following a period of RR disease. PPMS affect approximately 15% of people with MS where the neurological deterioration is present from the onset, most frequently without superimposed relapses. The rare variant where a few acute exacerbations are superimposed on the gradual PPMS-like course is called progressiverelapsing MS (PRMS) [7]. This study is focused on comparing urinary symptoms and urodynamic findings in RR and SP MS as there is currently not much known in the area of research.
Urinary symptoms are very prevalent among MS patients but there are little data on whether urinary differences exist among gender or disease subtype. In 2010, a cross sectional survey found that 65% of MS patients reported moderate to severe urinary symptoms and 26-37% reported using some type of urinary catheter to manage for bladder symptoms [8,9]. However, in this population based cohort, there were no differentiation of urinary symptoms by gender or subtype of disease and there was no urodynamic evaluation performed. Currently, there are consensus recommendations for urologic management of multiple sclerosis patients, which based on a generalized urinary phenotype [10], without specific recommendations for management of urinary symptoms per gender or disease subtype. Consequently, a better understanding of potential differences in urinary symptoms among gender and disease subtypes may lead to more specific and tailored patient care.
Given the limited available data, we sought to determine if urinary symptoms and urodynamic findings differed by gender and disease subtypes among multiple sclerosis patients referred to a neuro-urology clinic.
We conducted a retrospective review of a cohort of MS patients with symptoms of urinary incontinence, frequency, or retention who were referred for neuro-urologic evaluation over a 5 year period (2007-2012). All patients were diagnosed and treated at the University of Michigan Multiple Sclerosis Center. MS subtype (RRMS, SPMS) was categorized based on clinical history, exam and MRI diagnostic criteria [7]. All patients included this cohort had urological evaluation including urodynamic testing. If a patient had multiple urodynamic studies after initial urologic evaluation, data was utilized from first study. Frequency and severity of urologic symptoms were assessed using the American Urological Association Symptom Index (AUASI) [11] and Michigan Incontinence Symptom Index (M-ISI) [12] questionnaires administered at initial visit. Patients with total AUASI scores > 7 were considered to have moderate to severe symptoms. The AUASI was used to assess severity of irritative and obstructive urinary symptoms and the M-ISI questionnaire was used to assess severity of reported urinary incontinence from stress incontinence, urgency incontinence and pad use (M-ISI severity score). AUASI and M-ISI scores were excluded for patients with indwelling catheters (N=15). Charts were additionally reviewed for demographics, bladder specific pharmacologic treatments, use of intermittent or indwelling urinary catheters, and history of chronic urinary tract infections (>3 symptomatic episodes treated with antibiotics over a 6 month period).
All patients underwent standardized fluoro-urodynamic testing (FUDS) with complex cystometrogram, pressure flow studies, patch perineal electromyographic recording (EMG), and concomitant fluoroscopy. A 7 Fr dual chamber urethral catheter and 10 Fr rectal catheter were utilized to measure bladder and abdominal pressures. International Continence Society urodynamic and terminology guidelines were followed [13,14]. Detrusor over activity (DO) was defined as a symptomatic rise in detrusor pressure of >5 cm of during filling. Detrusor sphincter dyssynergia (DSD) was defined as sustained, involuntary increased EMG activity with or without characteristic radiographic findings proximal urethral dilation with intermittent, focal obstruction during voiding. Bladder compliance calculated as change in bladder volume/change in detrusor pressure on cystometrogram and low compliance was defined as <12.5 cc/cm H20 during bladder filling in the absence of a detrusor contraction [15]. Maximum voiding pressure was defined as Pdetmax, and was measured as the highest detrusor pressure when attempting to void regardless of urine flow. Maximum flows (Qmax) were unable to be consistently measured due to limitations related to patient positioning, such as limb contractures and body habitus which made it impossible to capture and measure the urine stream in a uroflowmeter.
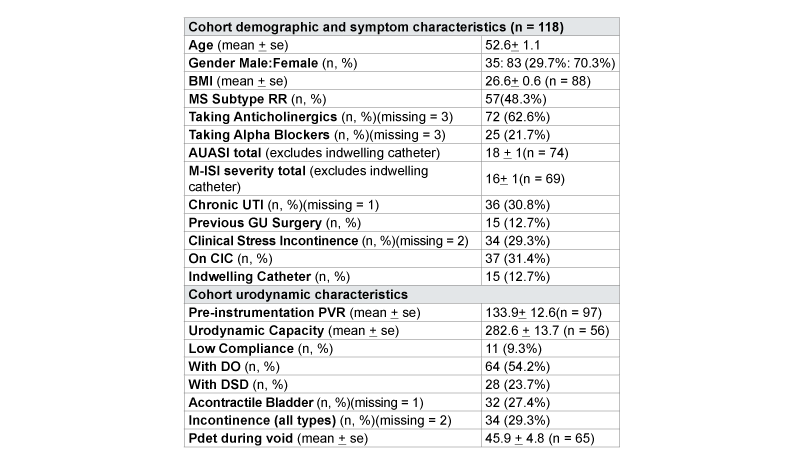
Data were extracted for 118 unique MS patients during the study timeframe. Demographic, history, urinary symptom and urodynamic data for the entire cohort are reported in Table 1. Mean AUASI and M-ISI severity scores were 18 (S.D.1) and 16 (S.D.1), respectively. Almost two thirds of patients were currently taking bladder medications (e.g. anticholinergics or alpha blockers), approximately half of the cohort used some type of bladder catheterization, and a third had a history of chronic urinary tract infections. Previous genitourinary procedures had been performed in 15 patients, including botulinum toxin injection, treatment for urinary calculus, cystoscopy, prostate biopsy, hysterectomy, urethral bulking injection, orchidopexy, and inguinal hernia repair. On urodynamic studies, 54% had DO, 24% had DSD, and 9% had low bladder compliance. Pdetmax during voiding was 46 cm of H20, for patients able to avoid during urodynamic testing (Table 1).
Gender analysis of urinary symptoms was performed on the 35 males and 83 females in the cohort (Table 2). AUASI scores were similar between men and women (19 vs.18, p=0.4) and there was a trending toward higher M-ISI scores in women, although not statistically significant (14 vs. 18, p=0.1). More men were taking alpha blocker medication compared to women (41% vs. 14%, p=0.001). There were otherwise no significant differences in demographics, use of chronic catheterization, use of intermittent catheterization, use of antimuscarinic medications, or number of patients with chronic UTI’s between gender cohorts. On urodynamic studies, males and females had similar findings of DO (males 57%, females 53%, p=0.7), DSD (males 29%, females 22%, p=0.4), and low bladder compliance (males 11%, females 8%, p=0.7). However, males had higher Pdetmax compared to females (63.9 vs. 36.1 cm H20, p=0.005).
Multiple sclerosis disease subtype analysis was performed for 57 RRMS and 61 SPMS patients (Table 3). RRMS patients had significantly higher mean AUASI scores compared to SPMS (21 vs.15, p=0.004) but similar M-ISI severity scores (16 vs.17, p=0.5). SPMS patients were also older at initial evaluation (52 vs. 47, p=0.0005), compared to RRMS, and used more indwelling catheters at initial evaluation (20% vs. 5%, p=0.02). There were no differences in prevalence of chronic urinary tract infections or urodynamic findings between subtypes.
In multiple regression models examining the relationships found on univariate analysis, gender was the primary predictor for Pdetmax (p=0.003) and RRMS subtype was associated with moderate to severe symptoms reported on AUASI independent of age and gender (OR 17.1, p=0.008). Subtype of MS did not significantly influence Pdetmax independent of age, gender (p=0.4) (Table 4). We did not find a significant impact of MS duration on outcomes nor did it confound our observed relationships between type and urinary symptoms, however, we were limited in our sample size.

Table 1: Characteristics by of Multiple Sclerosis Cohort Grouped by Disease Subtype
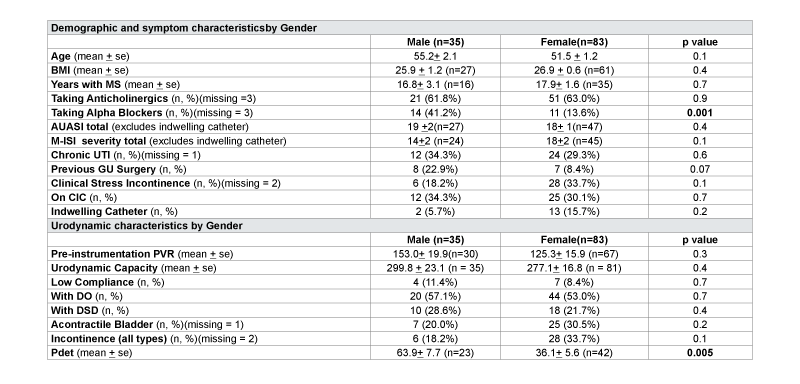
Table 2: Characteristics by of Multiple Sclerosis Cohort Grouped by Gender
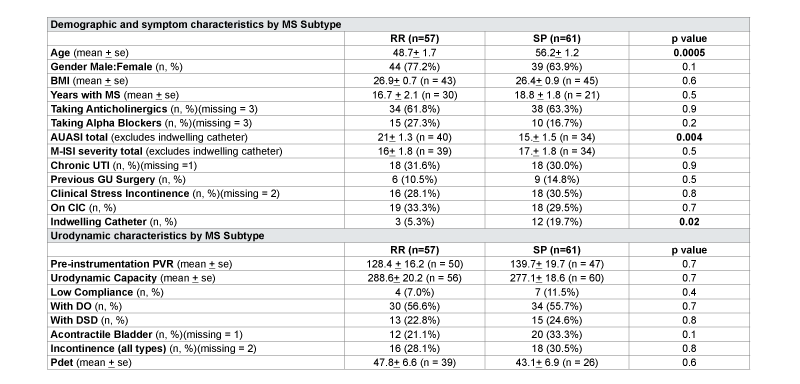
Table 3:Characteristics by of Multiple Sclerosis Cohort Grouped by Disease Subtype
Urinary symptoms can greatly impact quality of life in multiple sclerosis patients and up to 50% of MS patients can experience frequent episodes of urinary incontinence [8,16,17]. The 118 MS patients in our cohort also demonstrated moderate to severe urinary symptoms, as evidenced by elevated AUASI (mean score 18) and M-ISI severity (mean score 16) scores for the cohort. When looking at differences by gender between these MS patients, we found that males and females reported similar severity of urinary symptoms and had similar urodynamic findings, with the exception of higher voiding pressures in males. When examining by MS subtype, RRMS subtype was independently associated with increased odds of having moderate to severe AUASI scores despite having similar urodynamic findings. There were other few urinary differences between gender and disease subtypes in this MS cohort.
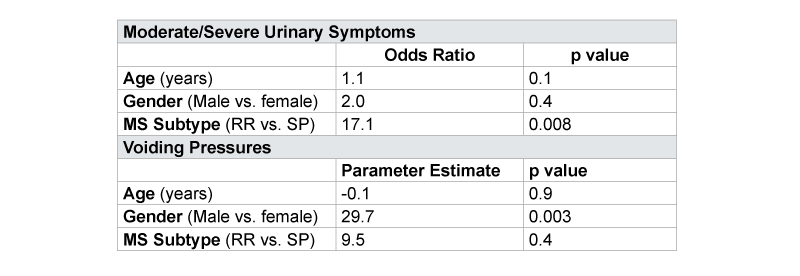
Table 4:Multivariable Analysis for Association between MS Subtype and Urinary Symptoms and Voiding PressuresAdjusted for Age and Gender
Male patients in this study more frequently took alpha blocker medications, and still had significantly higher voiding pressures on urodynamics compared to females. Our observations regarding the differences between genders in MS are similar to those reported by Giannantoni in a cohort of 116 Italian men and women with MS [18]. However, the observed urodynamic voiding pressure differences (mean Pdetmax in men=63.9 cm H20 and in women =36.1 cm H20) in our cohort are also similar to the age specific voiding pressure ranges in nonMS populations. Rosario reported Pdetmax ranged between 27-83 cm H20 for males aged 35-87 and Mahfouz noted a Pdetmax range of 22- 46 cm H20 in asymptomatic women [19,20]. Since both genders in our MS cohort had similar prevalence of detrusor sphincter dyssynergia on urodynamics, the presence of a prostatemay have influenced the observed differences between male and female voiding pressures in our cohort. The multivariable analysis finding only gender associated with higher voiding pressures gives strength to this possibility. However, since there is little information in the literature on how to best manage male MS patients with obstructive voiding symptoms, future prospective research is needed to determine if aggressive pharmacologic or surgical therapy targeted at reducing prostatic obstruction would beneficial in male MS patients with urinary symptoms, and high voiding pressures but no DSD on urodynamics.
Our cohort did not demonstrate a significant difference by gender in prevalence of potentially dangerous urinary conditions, such as DSD, low bladder compliance, or chronic urinary tract infections; though a previous study reported that female gender may be a risk factor for increased urinary complications in patients with MS [21]. Comparing morbidity between studies is challenging due to patients having differing access to medical care, urologic follow up, and unknown additional confounders. Our results suggest that both male and female MS patients are equally prone to have urodynamic dysfuctions. An international multi-institutional prospective study, with uniform treatment pathways and follow up, would help determine if gender is independently associated with increased risk of urologic complications in MS patients over time.
Comparing urinary symptom differences among MS disease subtypes, RRMS patients in this cohort reported higher overall AUASI compared to SPMS patients. After accounting for age and gender, patients with RRMS had a 17 fold greater odds of having moderate/severe LUTS than patients with SPMS, though there were no differences in severity of urinary incontinence (M-ISI severity) or urodynamic findings between the subtypes. Overall the urodynamic dysfunction is equally prevalent in both RR and SP-MS patients. This is consistent with a recent report on high prevalence of urinary symptoms and urodynamic dysfunctions in patients with clinically isolated syndrome (CISs) [22]. CIS is the first clinical presentation of MS and precedes the diagnosis of RR-MS. Despite of few MRI lesions at the diagnosis, 53.6% CIS patients reported urinary symptoms [22]. It is possible that CIS and RR-MS patients are more likely to report any new changes including urinary symptoms than SP-MS patients as the latter group of patients may have adapted to their symptoms. Alternatively, it is worth considering that the observed reduced bother from urinary symptoms in secondary progressive multiple sclerosis patients may be related to cognitive decline and decreased mobility. Bergendal reported that SPMS is associated with increasing risk
of cognitive decline compared to RRMS [23] and Raimonoted that apathy is also more prevalent in MS patients with severe disease [24]. Given the retrospective nature of our study, we were unable to assess cognitive or overall disability status of our patients to verify differences. Our study and others suggest that the bother from urinary symptoms are present and prevalent early on in MS disease course. This likely reflects the intrinsic MS disease activity in early MS patients. We propose to conduct early prospective urodynamic studies and administer urinary specific quality of life questionnaires to understand how RRMS patients perceive urinary symptoms compared to SPMS and how urinary symptoms change over time within each subtype in the future.
We noted that both MS subtypes groups had a similar prevalence of low bladder compliance (9%) and chronic urinary tract infections. Although others have reported an increase of low bladder compliance in MS patients with more advanced disease [25], our cohort is similar to Fletcher [26], who demonstrated a small incidence of low bladder compliance in 89 MS patients who were followed for a mean of 61 months. While we suggest that the incidence of low bladder compliance is likely low, the role and timing of urodynamic testing is not optimally defined for MS patients.
We recognize limitations associated with the retrospective nature of this study. One limitation is that our analysis was confined to MS patients referred to a tertiary neuro-urology center for evaluation of urinary symptomatology. We acknowledge that many MS patients have minimal lower urinary tract symptoms which do not require urodynamic testing for clarification and therefore, our cohort represents a group of MS patients which may not be representative of all MS patients with urinary symptoms. However, our MS cohort is similar to other populations described in the urologic literature when comparing urodynamic findings and catheter usage. Litwiller performed a meta-analysis of 1882 MS patients [27] and found DO and DSD in 62% and 25% of urodynamic studies respectively. This is similar to the 54% and 23% prevalence of DO and DSD in our cohort. Mahajan found that 26-37% of MS patients responding to the cross-sectional NARCOMS survey utilized urinary catheters, which likewise compares similarly to the 43% of patients using catheters in our cohort [8,9]. Second, we acknowledge that sample size limited our ability to differentiate small differences in variables between gender and subtype groups, including subdomains of the AUASI and M-ISI. Despite this sample size limitation, we were able to demonstrate a significant difference in AUASI scores between subtype, indicating that our study was adequately powered to detect a large difference. Finally, the data in the study represents a cross sectional analysis of MS patients presenting for evaluation. Consequently, we do not have AUASI or M-ISI scores prior to the first urology visit or before indwelling catheters were placed. These questionnaires could be implementing earlier in MS neurology clinic to better capture initial symptom data on MS patients for future studies.
Currently, the recommendations for testing and treating MS patients with urinary symptoms are very broad [28-30]. Our study adds to clinical knowledge by demonstrating that perception of severity of urinary symptoms may differ between MS subtypes, despite similar quantity of incontinence and, prevalence of urodynamic findings. Additionally, our data suggests that male MS patients have higher voiding pressures on urodynamics likely related to prostatic obstruction rather than DSD or bladder compliance changes. These data offer a starting point for further investigation on how MS patients perceive symptoms and whether anatomic differences between genders potentially open different treatment strategies for MS patients suffering from urinary symptoms.
In this cohort of MS patients, both male and female MS patients had similar urinary symptoms on questionnaires and similar urodynamic findings, with the exception of higher voiding pressures in males. Since both genders in our MS cohort had similar prevalence of detrusor sphincter dyssynergia on urodynamics, male patients may have higher voiding pressures due to the presence of a prostate rather than neurogenic voiding dysfunction. Although RR and SP MS had urodynamic findings, demonstrating a similar prevalence of DO (average 54.2%), DSD (23.7%), acontractile bladder (27.4%), and low bladder compliance (9%), RRMS subtype was independently associated with greater odds of having moderate/severe urinary symptoms. Exploring early perception and anatomical differences between subgroups of multiple sclerosis patients could lead to new avenues for treatment.
Download Provisional PDF Here
Article Type: Research Article
Citation: Cox L, Cameron AP, Wittman D, Papin JE, Mao-Draayer Y, et al. (2015) Analysis of Urinary Symptoms and Urodynamic Findings in Multiple Sclerosis Patients by Gender and Disease Subtype. J Neurol Neurobiol 1 (2): http://dx.doi.org/10.16966/2379-7150.105
Copyright:© 2015 Cox L, et al. This is an openaccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access