Abstract
Objective: Imaging findings of cerebrovascular dysfunction following endovascular therapy have been increasingly reported. Special attention has been given to leptomeningeal abnormalities seen on post-contrast FLAIR (pcFLAIR) imaging as its presence is associated with poorer prognosis following endovascular therapy. The aim of this study is to investigate the relationship of various clinical variables on the presence and extent of pcFLAIR abnormalities in patients undergoing elective flow diversion of intracranial aneurysms.
Patients and Methods: In a single center retrospective cohort, 80 consecutive patients treated with flow diverting stents who subsequently underwent post-operative MR-imaging within 24 hours of the procedure between July 2012 and November 2017 were identified and included in the study. Patient, aneurysm, and imaging characteristics were collected for review. Analysis of clinical variables on the presence and extent of leptomeningeal abnormalities seen on pcFLAIR imaging was performed.
Results: We identified 69 of 80 patients (86%) with leptomeningeal enhancement (LME) on pcFLAIR imaging. In all instances, the distribution of LME matched the ipsilateral side of endovascular intervention. Of the 20 patients who had both pre and pcFLAIR imaging, LME was absent on precontrast imaging and seen exclusively on pcFLAIR in all cases. Complete resolution of LME was seen in 31 of 35 (89%) patients with available followup imaging. No significant association was found between LME and the extent of DWI abnormalities, dose of intra-arterial contrast administration, anesthetic or opioid use, procedural time, time until post-operative MR imaging, renal function, age, or number of stents used during embolization. There is a significant inverse relationship between the presence and extent of LME and the dose of intra-operative midazolam administration used during anesthesia (p=0.018).
Conclusion: The majority of patients undergoing flow diversion therapy will develop LME on pcFLAIR imaging. LME is absent on pre-contrast imaging and seen exclusively on pcFLAIR in all cases. LME appears to be a transient finding affecting the vascular territory of intervention but in some cases may take months to completely resolve. There is no direct relationship of LME with lesions on DWI or the timing of MR imaging but there is a statistically significant inverse association of midazolam administration and presence and extent of LME. This study provides new evidence suggesting that midazolam may reduce the presence and extent of LME following neuroendovascular surgery and could have neuroprotective effects.
Keywords
Aneurysm; Contrast-induced neurotoxicity; Blood brain barrier; Endovascular therapy; Intracranial aneurysms; Cerebrovascular procedures; Leptomeningeal enhancement
Abbreviations
LME-Leptomeningeal Enhancement; PcFLAIR-Post p Contrast Fluid-Attenuated Inversion Recovery
Introduction
Contemporary management of intracranial aneurysms is now largely dominated by endovascular techniques [1,2] requiring complex and technically advanced endoluminal manipulations. The increasing technical complexity of these procedures is not without risk and complications. While complications such as subarachnoid hemorrhage and thromboembolism are well-described, the effect of endoluminal manipulations and intra-arterial medications on downstream cerebrovascular structures following endovascular therapy remains largely unknown. Imaging biomarkers of cerebrovascular dysfunction following endovascular therapy have been reported, including leptomeningeal hyperintensity on CT [3] and leptomeningeal enhancement (LME) on pre- and postcontrast FLAIR MR imaging [1,4] . LME on post-contrast FLAIR (pcFLAIR) imaging following acute stroke intervention has been touted as an indicator of blood-brain barrier (BBB) breakdown and portends a poorer prognosis with increased rates of reperfusion injury and neurological deficits [5,6]. However, LME is not strictly associated with cerebrovascular disease as it has also been reported with normal aging [7], also attributed to diminished BBB integrity. LME on pcFLAIR has been increasingly identified and targeted as an important imaging biomarker following endovascular surgery due to its prognostic value after stroke intervention, but the incidence and pathophysiology of LME remain unknown [8]. We previously found a significant relationship between pcFLAIR enhancement and development of post-procedure neurological deficits in a small series of patients who underwent flow diversion therapy [9]. The aim of this study is to investigate the relationship of various clinical variables on the development of pcFLAIR abnormalities in patients undergoing elective flow diversion of intracranial aneurysms.
Patients and Methods
Subjects
In this hospital Institutional Review Board and Health Insurance Portability and Accountability Act-approved single-center retrospective study, consecutive patients treated with flow diverting stents between July 2012 and November 2017 was identified. A total of 80 patients subsequently underwent post-operative MR-imaging within 24 hours of the procedure and were included for further review. pcFLAIR sequences were intermittently included in post-operative head MR imaging up until 2016, where upon pcFLAIR imaging was incorporated as the standard FLAIR imaging pulse sequence at our institution. Informed consent for endovascular treatment and imaging examinations were obtained from the patient in all cases.
Procedure
All endovascular procedures were performed on a bi-plane angiographic unit with the patient under general anesthesia. Systemic blood pressure was maintained at systolic pressures of between 90 and 140 mmHg. Phenylephrine or ephedrine were used to maintain systolic blood pressures above target. Iodixanol 300 (GE Healthcare, Little Chalfont, and United Kingdom) contrast was used during cerebral angiography in all but one patient. Verapamil was infused intra-arterially for either prevention or treatment of catheterassociated vasospasm. Systemic heparinization was administered to a target ACT of 200-300. Contrast administration was performed by a hand injection technique utilizing between 4-6 ml total volumes of contrast per parent vessel injection. Standard guide catheters and stent deployment micro catheters were used for flow diverting stent placement. Aneurysm embolization was performed in the standard fashion with stent deployment across the neck of the aneurysm. All patients included in this study completed the embolization procedures with no intra-operative complications, including intra-luminal thrombus formation or aneurysm perforation.
Imaging
The MR brain imaging protocol consisted of a comprehensive brain MRI with DWI, pre- and post-contrast T1 imaging, non-contrast time-of-flight MR angiography, post-contrast 3D sagittal T2-weighted FLAIR imaging pulse sequence (TR/TE/TI 6000ms/135ms/1700ms, slice thickness 1.6 mm, matrix 256 × 256). Contrast-enhanced FLAIR was acquired after a dose of 0.1 mmol/kg intravenous gadobenate dimeglumine contrast (Multihance, Bracco Diagnostics, and Princeton, NJ)..
Image analysis
All acquired images were stored on and reviewed from our PACS imaging server. Pre- and pcFLAIR sequences in axial and coronal planes were read independently by one neuroradiologist (DS) and one neurological surgeon (YL) with both blinded to the patient and to the presence of other clinical factors. The extent of LME was categorized as focal (involving a single or a few sulci but less than half of a lobule or cerebellar hemisphere), lobar (involving a majority of the frontal, parietal, temporal, or occipital lobe or hemisphere of the cerebellum), or hemispheric (involving more than half of at least two out of four lobes or both cerebellar hemispheres). The location was documented according to conventional arterial territory (right/ left anterior cerebral artery, right/left middle cerebral artery, right/left posterior cerebral artery, and right/left cerebellar hemispheres) and compared to the site of intervention. DWI was evaluated for lesions reflecting the presence of acute infarction, which was defined as high signal intensity with corresponding low signal intensity of ADC. The number of lesions was then graded according to previously published grading scales [10]: as none, grade 1 (<6 foci of lesions), and grade 2 (> or equal to 6 foci of lesions) CT imaging immediately post-procedure (when available) was reviewed for presence of LME and correlated with pcFLAIR imaging. Follow-up MR imaging studies performed 2 weeks-19 months following the index procedure were evaluated (when available). Descriptive statistics, univariate, and multivariate regression statistical analysis were performed.
Statistics
Statistical values were calculated using excel data analytics software (Microsoft, Redmond, WA). Univariate and multivariate logistic regression analysis was performed using SAS analytics software (SAS Institute, Cary, NC). Professional statistical consultation was obtained with the department of biostatistics at the university of WisconsinMadison. Statistical significance was defined as a P<0.05. All variables with a P<0.10 were included in a multivariate logistic regression. Odds ratios (ORs) and 95% confidence intervals (CIs) are presented for statistically significant findings where applicable.
Results
Patient characteristics
We identified and evaluated 80 patients who underwent endovascular flow diversion of 84 intracranial aneurysms between July 2012 and November 2017. Our cohort was predominantly female, which reflects the overall gender predilection [11]. The mean age at treatment was 56.8 ± 13 years. No patients in our cohort had subarachnoid hemorrhage before or after endovascular treatment. Embolization was performed using 1.3 ± 0.4 stents. Aneurysm size ranged from 2-25 mm in diameter with a mean of 6.1 ± 4.1 mm. In total, 37 patients presented with hypertension and 23 with history of tobacco use. No significant associations were found between LME and patient or aneurysm characteristics. Descriptive statistics are presented in table 1.
| |
(-) LME pcFLAIR |
(+) LME pcFLAIR |
p value |
Total |
| Number of patients |
11 |
69 |
|
80 |
| Sex |
1 M/10 F |
7 M/62 F |
|
8 M/ 72 F |
| Age (years)a |
58 ± 16.2 |
56.6 ± 12.6 |
0.74 |
56.8 ± 13.1 |
| Aneurysm size (mm)a |
6.5 ± 7.4 |
6.0 ± 3.4 |
0.71 |
6.1 ± 4.1 |
| No. stents |
1.2 ± 0.4 |
1.3 ± 0.4 |
0.44 |
1.2 ± 0.4 |
| Lesion on DWI |
2 (18.2%) |
26 (37.7%) |
|
28 (35%) |
| Verapamil (mg)a |
4.7 ± 3.6 |
3.9 ± 3.9 |
0.53 |
4.0 ± 3.8 |
| Dose of IA contrast (mg)a |
96 ± 29 |
102 ± 38 |
0.62 |
101 ± 37 |
| Presumed contrast staining on CT |
0 (0%) |
11 (35%) |
|
11/35 (31%) |
| Diabetes |
4 (36%) |
16 (23%) |
|
|
| Hypertension |
5 (45.5%) |
32 (46.4%) |
|
37 (46.3%) |
| Smoking |
3 (27.3%) |
20 (29%) |
|
23 (28.8%) |
| BMI |
29.2 ± 5.4 |
31.1 ± 6.8 |
0.38 |
|
| ASA class |
2.5 ± 0.6 |
2.6 ± 0.5 |
0.55 |
|
| aprovided as mean ± SD. |
|
|
|
|
Table 1: Patient demographics and imaging findings.
aprovided as mean ± SD.
LME incidence and location
In our cohort 69/80 (86%) of patients developed abnormal LME on post-operative pcFLAIR imaging. Of the 84 aneurysms, the location of the target aneurysm was as follows: superior hypophyseal (n=30), supraclinoid (n=32), vertebral/basilar artery (n=10), distal anterior cerebral artery circulation (n=12), distal middle cerebral artery circulation (n=0). In all instances, the distribution of LME matched the site of intervention (Figure 1). In 6 cases, two of which included embolization of anterior cerebral artery aneurysms and four involving the internal carotid artery, there was bilateral LME of the anterior cerebral artery territory due to robust communicating arteries. Of the 20 patients who had both pre- and pcFLAIR imaging, LME was absent on pre-contrast imaging and seen exclusively on pcFLAIR in all cases (Figure 2). A total of 35 (44%) patients also underwent CT imaging immediately post-procedure. Presumed subarachnoid contrast staining was observed in 11 of 35 patients (31%), with all 11 patients also demonstrating pcFLAIR LME. Complete resolution of LME was seen in 31 of 35 (89%) patients with available follow-up imaging. In one case LME persisted at 3 months but resolved at 19 months. Of the remaining 3 patients, delayed follow-up imaging was not available to confirm resolution of LME. Of the 4 cases in which LME did not resolve, a reduction in extent of abnormal enhancement was noted in all instances on available follow-up imaging
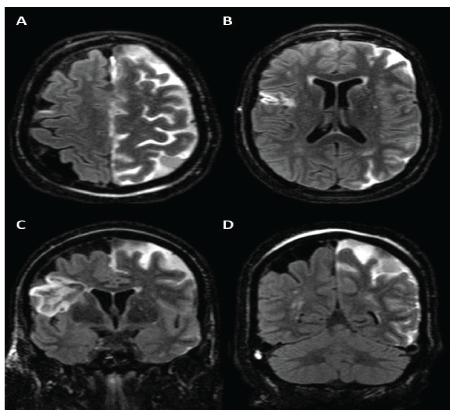
Figure 1: (black and white) 74-year-old woman following Pipeline embolization of a dysplastic left ophthalmic segment internal carotid artery aneurysm. Axial (A, B) and coronal (C, D) MRI post contrast FLAIR sequences demonstrating hemispheric leptomeningeal T2/FLAIR signal hyper intensity. Note there is laminar necrosis in the right frontal lobe from an evolving prior chronic infarct.
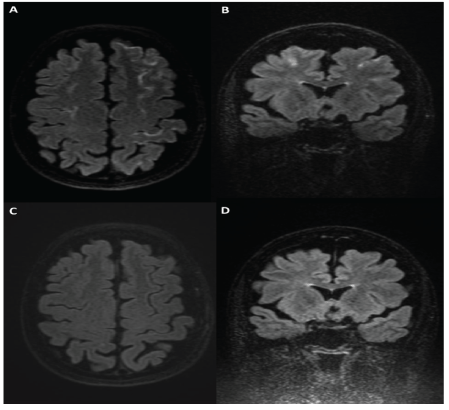
Figure 2: (black and white) 58-year-old man following flow diversion treatment of a left ophthalmic segment internal carotid artery aneurysm. Axial and coronal (A, B) post-contrast FLAIR imaging demonstrating lobar leptomeningeal enhancement that is absent on pre-contrast FLAIR imaging (C, D).
LME and timing to MR imaging
There was no significant association between presence or extent of LME and the timing of post-operative MR imaging. In patients with absence of LME, the average time from conclusion of intervention to MR imaging was 684 ± 549 minutes compared to 519 ± 351 minutes, 440 ± 295 minutes, and 719 ± 593 minutes for focal, lobar, and hemispheric LME respectively (p=0.11).
LME and DWI lesions
There was no significant association between the presence of DWI lesions and the presence of LME (p=0.65). Similarly, there was no significant relationship between grading of DWI lesions and the grading of LME (p=0.22). In our patient series, 35% of patients demonstrated lesions on DWI with 6% occurring bilaterally. No patient had evidence of cortical or subcortical infarct volume larger than 0.5 cm3. In total, 117 lesions were identified on DWI: 26 in patients without LME; 28 with focal; 30 with lobar; and 33 with hemispheric grades of LME. The majority of DWI lesions were in the MCA territory (69%) followed by ACA (16%), PCA (2%), and cerebellum (2%). Grade 1 DWI lesions were identified in 21% of patients while 7% demonstrated grade 2 lesions.
LME and intravenous midazolam
A significant inverse relationship was identified on univariate and multivariate analysis between the administration of midazolam and the presence and extent of LME (p=0.01). In patients with absence of LME, 10/11 (91%) were administered midazolam compared to 21/25 (84%), 20/34 (59%), and 4/10 (40%) demonstrating focal, lobar, and hemispheric enhancement of pcFLAIR imaging respectively (Table 2).
| |
Odds Ratio |
95% CI |
p value |
| Univariate |
| Sex |
1.15 |
0.30-1.20 |
0.97 |
| Diabetes |
0.53 |
0.14-2.03 |
0.35 |
| Hypertension |
1.03 |
0.28-3.72 |
0.95 |
| Smoking |
1.09 |
0.26-4.52 |
0.9 |
| Presence of DWI |
2.72 |
0.55-13.6 |
0.18 |
| Verapamil |
1.21 |
0.75-1.10 |
0.77 |
| Dose of Contrast |
1.02 |
0.76-1.25 |
0.12 |
| Rocuronium |
0.28 |
0.72-1.18 |
0.32 |
| Sevoflourane |
1.02 |
0.86-1.39 |
0.69 |
| Midazolam |
0.37 |
0.34-0.77 |
0.01 |
| Propofol |
1.01 |
0.92-1.12 |
0.46 |
| Fentanyl |
0.72 |
0.70-1.62 |
0.9 |
| Multivariate |
| Midazolam |
0.31 |
0.19-0.82 |
0.018 |
Table 2: Univariate and multivariate analysis of associations between clinical variables and pcFLAIR LME.
LME and other anesthetics/medications
There was no significant association between the administration of rocuronium, sevoflurane, propofol, fentanyl, iodinated contrast, or verapamil and the presence and extent of LME (Table 2). The average dose of verapamil was 4.0 ± 3.8 mg. In the group positive for LME, the average dose was 3.9 ± 3.9 mg compared to 4.7 ± 3.6 mg in the negative group (p=0.53). The average dose of iodinated contrast (Visipaque 320, an iso-osmolar iodinated contrast agent) was 101 ± 37 mg. In the group positive for LME, the average dose was 102 ± 38 mg compared to 96 ± 29 mg in the negative group (p=0.62).
Discussion
Flow diversion therapy is associated with LME on pcFLAIR imaging
We previously found a significant relationship between pcFLAIR enhancement and development of post-procedure neurological deficits in a small series of patients who underwent flow diversion therapy [9]. The current study focuses on the relationship between various clinical variables and the presence and extent of LME on postoperative imaging following neuroangiographic procedures. Here, we observed LME in 86% of patients undergoing flow diversion of intracranial aneurysms. Sulcal hyperintensity on CT or LME on MR imaging following neuroendovascular intervention is an increasingly recognized radiographic phenomenon. Subarachnoid contrast staining has been reported on immediate post-operative CT in up to 56% of patients undergoing endovascular procedures. In all cases, complete resolution of leptomeningeal hyperintensity was noted within 4-6 hours of the procedure [3]. In this study, no abnormalities were seen on non-contrast FLAIR imaging; however, pcFLAIR imaging was not done [3]. In our cohort a total of 35 (44%) patients also underwent CT imaging immediately post procedure. Presumed subarachnoid contrast staining was observed in 11/35 patients (31%) consistent with previous findings. The incidence of LME on pcFLAIR imaging following endovascular stroke treatment ranges from 33-45% [5,12]. LME was also reported in a small case series of 12 patients undergoing carotid stent placement; in this study, all 12 patients developed LME [13]. More recently, LME was observed post-operatively in 52% of patients undergoing uncomplicated embolization of intracranial aneurysms [4]. In this series, the authors performed MR imaging within 72 hours of the procedure with an average time of 16.2 hours ranging from 3.9 hours to 64.3 hours delay. There was no significant difference found between timing of imaging and the presence of LME. In all cases, the authors reported complete resolution of LME with delayed follow-up imaging (27/62 with delayed imaging). The timing of delayed imaging was not reported. In our cohort the average time between conclusion of the procedure and MR imaging was 8.8 hours with a range of 1.8-22.3 hours. We also did not identify a significant association between the presence or extent of LME and the timing of imaging. In our cohort, resolution of LME was seen in 31 of 35 (89%) patients with available follow-up imaging with an average follow-up period of 3.8 months ranging from 0.5-19 months. In one case LME persisted at 3 months but resolved at 19 months. The other three patients without resolution in initial follow-up imaging did not have subsequent imaging to confirm resolution of LME; however, all cases demonstrated decrease in extent of LME compared to initial post-operative imaging suggesting that resolution of LME is a timedependent process. The average time of follow-up imaging for the three cases without resolution of LME was considerably earlier than in the cohort in which LME was found to be resolved (1.7 months compared to 3.8 months). The higher incidence of LME observed in our series relative to previously published series may attributable to earlier imaging as resolution of LME appears to be a time-dependent process. Earlier imaging may identify a higher number of patients with focal degrees of LME that would resolve with delayed scans. We hypothesize that LME likely resolves over days rather than hours which may account for the lack of association between the timing of initial MR imaging and the presence and extent of LME found in our series. LME was seen within the ipsilateral circulation in all cases and was seen bilaterally in 6 patients with robust communicating arteries. In these 6 cases, two of which included embolization of anterior cerebral artery aneurysms and four involving the internal carotid artery, there was bilateral LME of the anterior cerebral artery territory. This is consistent with previous reports where LME was observed in arterial territories exposed to endovascular surgery during the procedure or within the vascular territory perfused by the parent vessel [4]. These findings suggest a direct effect on downstream vasculature during intervention rather than a systemic process that would contribute to global cerebrovascular dysfunction. The differential diagnosis of ipsilateral LME on pcFLAIR following endovascular therapy is very broad. FLAIR is particularly sensitive in the evaluation of subarachnoid and periventricular pathology [14]. Acute subarachnoid hemorrhage, which is the most devastating complication of neuroendovascular interventions, can present as leptomeningeal signal on both pre-contrast and pcFLAIR. Rates of procedure-related subarachnoid hemorrhage due to aneurysm rupture can be as high as 16% and minor hemorrhages may occur even without clinical or angiographic evidence [15]. Of the 20 patients in our cohort who had pre- and pcFLAIR imaging, we did not observe evidence of subarachnoid hemorrhage on pre-contrast FLAIR, so it is unlikely that LME on pcFLAIR reflects hemorrhage. As previously discussed, sulcal hyperintensity on CT or LME on MR imaging following neuroendovascular intervention is an increasingly recognized radiographic phenomenon hypothesized to be due to disruption of the BBB [3,4]. In these series, LME was only appreciated on post-contrast imaging, consistent with the findings reported above. In our cohort, all patients who were found to have contrast staining on CT also had LME on pcFLAIR. However, it is also worth noting that approximately two thirds of patients who had LME and underwent CT did not have contrast staining, thereby suggesting that pcFLAIR is a more sensitive and potentially superior imaging biomarker for cerebrovascular and BBB dysfunction than contrast staining seen on CT. Moreover, our observation that none of the 20 patients with both pre-contrast and pcFLAIR had LME on pre-contrast FLAIR supports BBB hyperpermeability to gadolinium as the most likely mechanism of LME following endovascular intervention. BBB hyperpermeability could be the result of intra-arterial medications or temporary ischemia induced by cerebrovascular manipulations during endovascular intervention [16]. We sought to further investigate the effect of patient and clinical factors such as medications, anesthetics, and presence of ischemia noted on DWI with the incidence and extent of pcFLAIR abnormalities. Univariate and multivariate analyses were performed and the results presented in table 2.
LME is not associated with lesions identified on DWI
One potential mechanism of LME could be cerebrovascular dysfunction induced by diffuse micro-embolisms. Cerebral ischemia has been shown to result in disruption of the BBB seen early after acute infarction manifesting as LME on pcFLAIR imaging [4,11]. Klotzch et al. identified micro-embolisms on transcranial Doppler ultrasonography in 31% of patients undergoing neuroendovascular surgery [17]. A separate group reported direct correlations of emboli identified on transcranial doppler ultrasonography and the presence of new DWI lesions after carotid surgery [18]. The presence and extent of lesions on DWI may therefore reflect the degree of ischemia induced upon the distal cerebral vasculature resulting in hyperpermeability of the BBB. DWI is a very sensitive technique for detecting ischemic lesions in the brain, particularly for smaller lesions that may be missed on other imaging modalities [19]. The sensitivity and specificity of DWI for detecting cerebral ischemia has been reported between 90-98% and 100% respectively [19,20]. On univariate and multivariate analysis, our study failed to demonstrate a significant correlation between the presence and extent of LME and the manifestation or grading of lesions on DWI. These findings are consistent with previous reports suggesting alternative mechanisms may facilitate BBB disruption [4]. The average number of lesions per patient without LME was 2.4 ± 5.7 compared to 0.9 ± 1.6 for focal, 0.8 ± 1.7 for lobar, and 3.3 ± 3.3 for hemispheric degree of enhancement. There were no large-volume territorial infarctions encountered in our cohort. A total of 35% of patients in our cohort demonstrated lesions on DWI, whereas 86% demonstrated LME on pcFLAIR. Altogether, these analyses confirm that DWI abnormalities do not account for the occurrence of LME on pcFLAIR in our cohort. Nonetheless, it remains possible that small micro-emboli could be occult on DWI yet still produce disruptions of the BBB that manifest as LME on pcFLAIR imaging. We did not directly measure micro-emboli during endovascular surgery so we cannot exclude this possibility. If the presence of micro-emboli was found to correlate with LME, this would suggest that pcFLAIR is more sensitive than DWI for detecting micro-embolic insults following neuroendovascular surgery.
Midazolam is protective of developing LME on pcFLAIR imaging
Univariate and multivariate analyses were performed to identify the relationship between various patient demographics, clinical factors, and the administration of verapamil, iodinated contrast, rocuronium, sevoflurane, propofol, fentanyl, and midazolam during neuroendovascular surgery on the presence and extent of LME. Of the medications evaluated, a significant inverse relationship was identified between the administration of midazolam and the extent of LME. Midazolam is a short-acting benzodiazepine which activates GABAA receptors to increase Cl- channel opening within the central nervous system. Opening Cl- channels leads to neuronal hyperpolarization. Clinically, benzodiazepines promote sleep, sedation, and relaxation. As a result, midazolam is frequently used in the clinical setting for its anticonvulsant and anesthetic properties. GABAergic neurons regulate central inhibitory activity via Cl- channels which have been implicated in neuronal cell death [21,22]. Hyperpolarization induced by the opening of Cl- channels suppresses the excitatory amino acids triggered by cerebral ischemia [21,22], suggesting that activation of GABA receptors may therefore have a neuroprotective effect on the central nervous system. Midazolam has been shown to be protective against ischemia-induced neuronal injury [23,24]. For instance, midazolam was shown to inhibit the apoptosis of astrocytes by regulating apoptosis-related protein expression in vitro [24]. Astrocytes are essential for the formation and preservation of the BBB. Astrocytes secrete proteins that facilitate the formation of tight junctions which are vital in regulating the exchange of molecules in and out of the central nervous system. Neurological disorders such as cerebral ischemia, trauma, or neurodegenerative diseases all result in death of astrocytes and disruption of the BBB [25]. These findings provide a potential explanation for the inverse relationship between midazolam administration and presence of LME on pcFLAIR imaging observed in our cohort. Midazolam may act to prevent astrocyte apoptosis in the setting of local trauma or ischemia induced by neuroendovascular surgery. In our series, the dose of verapamil was not a significant contributor to the development of LME despite case reports of intra-arterial verapamil and papaverine inducing local disruptions in the BBB [26,27]. Vasodilators are often administered during neuroendovascular surgery when vascular vasospasm is induced by endoluminal manipulations using micro wires or catheters. During brief periods of cerebral ischemia, areas of penumbra maximally vasodilate and suspend autoregulation to improve local cerebral blood flow. These responses release local byproducts of cell lysis and increase BBB permeability. This hypothesis is further supported by reports of LME on pcFLAIR after carotid stenting for significant carotid stenosis in a cohort of 12 patients [13], a finding which the authors attributed to suspended autoregulation due to carotid stenosis and subsequent cerebral hyperperfusion post-stenting. In a different cohort after carotid stent placement, the incidence of LME was reported at 57% leading the authors to propose the etiology was most likely attributed to dysfunction of cerebral autoregulation and breakdown of the BBB [28]. In our cohort, hyperperfusion and cerebral dysautoregulation is less likely to be a contributing factor as no patient had significant parent vessel stenosis prior to stent placement. However, flow diversion may have temporary effects on the downstream cerebral vasculature but this phenomenon remains poorly understood [29]. BBB breakdown manifesting as LME is a reversible phenomenon that may be caused by a combination of localized endothelial injury by direct trauma, neurotoxic drugs, or ischemia associated with neuroendovascular surgery. Of all the major variables that were evaluated - including patient and aneurysm characteristics, anesthetics, and intra-arterial medications only midazolam demonstrated a statistically significant association and may be protective of developing imaging biomarkers of cerebrovascular dysfunction seen as present of LME.
Limitations
This retrospective study had several limitations. Most importantly, the retrospective design precluded us from including a control patient population of patients undergoing diagnostic cerebral angiography. Given that these patients rarely have post-procedure pcFLAIR imaging, control data from these patients would only be available as part of a prospective study. Additionally, this is a single center experience with a moderate cohort size. The MR imaging protocol did not include pre-contrast FLAIR imaging in the majority of patients; therefore, we cannot distinguish intrinsic FLAIR changes from gadolinium extravasation in the majority of cases. The total dose of iodinated contrast used was based on the total number of vials used which may therefore be an overestimation because it includes any residual contrast left within syringes. There are regional variations in patient populations, anesthetic use, and neuroendovascular techniques which may bias the incidence of LME on pcFLAIR. At our institution, we liberally use verapamil prior to selective catheterization of distal cerebral vessels to prevent cerebral vasospasm and therefore may have a higher incidence of verapamil administration compared to other institutions.
Conclusion
The majority of patients undergoing flow diversion therapy developed LME on pcFLAIR imaging. In patients who had both preand pcFLAIR imaging, LME was absent on pre-contrast imaging and seen exclusively on pcFLAIR in all cases, consistent with BBB breakdown. LME appears to be a transient finding affecting the vascular territory of intervention although in some cases it may take up to months to completely resolve. There is no direct relationship between LME and lesions on DWI or the timing of MR imaging. However, there is a statistically significant inverse association between midazolam administration and presence and extent of LME. This study provides new evidence suggesting that midazolam may reduce the presence and extent of LME following neuroendovascular surgery and could have neuroprotective effects.
Conflict of Interest
The authors do not have any competing interests to declare in regards to the publication of this manuscript.
Grants
JPY is supported by a Young Investigator Award from the Brain and Behavior Research Foundation and by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR002373. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The current study is not supported by any sources of funding. Abstract accepted for oral presentation at American Society of Neuroradiology, Vancouver, BC, 2018 as oral presentation.



