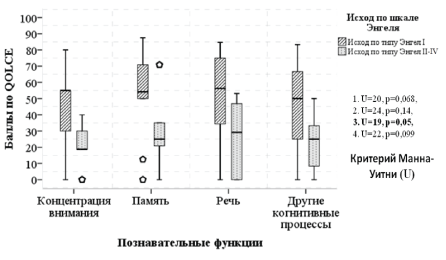
Figure 1: Outcome-specific distribution of post-surgical patients according to QOLCE questionnaire in Cognitive Function subscale.


Abramov KB Sarsembayeva DA Yu Ivanov A Odintsova GV Khachatryan WA Ivanova NE*
Polenov Russian Research Institute of Neurosurgery-Branch of Almazov National Medical Research Centre, St. Petersburg, Russia*Corresponding author: Ivanova NE, Polenov Russian Research Institute of Neurosurgery-Branch of Almazov National Medical Research Centre, St. Petersburg, Russia, E-mail: ivamel@yandex.ru
Objective: The objective was to assess the effect of surgical treatment of drug-resistant temporal lobe epilepsy and quality of life in children with drug-resistant epilepsy.
Methods and materials: We analyzed 80 case histories of children aged 2-17 with drug-resistant temporal lobe epilepsy, who had an operation in 2011-2016. Surgery outcomes were studied with Quality of Life in Childhood Epilepsy Questionnaire and Engel Epilepsy Surgery Outcome Scale.
Results and conclusion: In the long-term, 54.5% of children with drug-resistant temporal lobe epilepsy achieved Engel class of outcome after the operation, which lead to significant (p<0.05) positive changes in quality of life subscales Language, Physical Restrictions, Energy/Fatigue, Overall Quality of Life.
Temporal lobe epilepsy; Epilepsy; Epilepsy surgery; Quality of life; Children
Epilepsy is one of the most frequent and severe pediatric neurological disorders, occurring in 0, 9-2% of children [1,2]. Temporal lobe epilepsy accounts for approximately one third (30-35%) of all cases of epilepsy [3]. Even with the availability of drugs and using the optimal drug regimen, approximately 30% of patients do not respond to treatment [4,5], which leads to significant adverse social and economic effect for patients and their families [6].
It is believed that, despite the epileptogenic zone localness, the entire brain participates in epileptogenesis, and such an “epileptic brain” differs from a healthy one [7]. Child’s developmental disorder is associated with sometimes irreversible effect frequent seizures and antiepileptic drugs have on the developing brain, and often the etiology of epilepsy is not so important in this case [7-14]. There is a reasonable cause to consider a negative impact of the severity of seizures on the child’s psychosocial status to be proved [10- 13]. Cognitive functioning and behavior of children with epilepsy are impaired not only in the long-term course of the disease, but sometimes even before it is manifested [7]. Patients with the onset of epilepsy at an early age, in particular, with seizures that began before the age of two, are much more likely to be associated with cognitive and psychosocial function disorders, even if no intellectual disabilities are present [11,12,14]. Despite modern achievements in neurosurgery, very few patients are referred for surgical treatment [6,15,16]. Some authors call minimally-invasive epilepsy surgery “one of the most underutilized of all accepted medical treatments in the world” [16,17]. In a group of patients with epilepsy, especially pediatric, the most important aspects are seizure freedom and the need to take antiepileptic drugs, especially at an early age [18- 20]. Surgical treatment of drug-resistant form of the disease can become a generally accepted and affordable solution to achieve this goal [4,21,22]. Early seizure freedom correlates with a significant improvement in both the psychomotor development and the overall quality of life of the child [7,23].
Despite the increased number of studies concerning the epilepsy surgery in adults and children, the data are insufficient to generate high-strength recommendations (class I and II). Firm conclusions that can be drawn about the effect of surgery of drug-resistant forms of the disease on the cognitive and psychosocial spheres of life, especially in infants, are even fewer. Significant differences in the surgeries applied, questionnaires used to assess the quality of life and how the favorable surgery outcome is interpreted, make it quite difficult to compare the results obtained by researchers in different countries. The aim of this study was to assess the influence of surgical treatment on seizure frequency and quality of life in children with drug-resistant epilepsy in order to develop the optimal algorithm for screening of patients and selection of surgical approach.
We have performed the retrospective and prospective analysis of the results of examination and surgery of 80 children between 2 and 17 years of age (М=10.5 ± 0.45 years) with diagnosed drug-resistant temporal lobe epilepsy, of whom 41 (51.25%) were boys. Case history files contained data on examination and treatment results for each patient operated in the pediatric neurosurgery unit of Polenov Russian Research Institute of Neurosurgery within the period between 2011 and 2016 by one surgeon. Surgical treatment method was indicated in children for whom the complex examination helped localize the epileptogenic focus. Indications for surgical treatment were also 2 or more unprovoked epileptic seizures, frequent disabling epileptic seizures and a drug-resistant, progredient course of the disease. The criterion for epilepsy drug-resistance was the insufficient control or lack of seizure control during treatment with more than 2 first-line antiepileptic drugs for 18 months if the frequency of epileptic seizures persisted on average more than 1 attack per month for 18 months and more than 3-month seizure-free period was not achieved during that 18-month interval. In 2/3 cases, the duration of the disease exceeded 3 years. 32.5% of children had epilepsy for 7 years or more. The average duration of the disease was 6 years (Mo=7yrs). Surgical interventions were aimed at removing the epileptic focus (en bloc resection of the neocortical and/or paleocortical areas of the temporal lobe-51.25%) and/or removal of the structural changes focus (lesionectomy-32.5%). 6.25% of patients used multifocal subpial resections, 10% had other types of operations (endoscopic excision of the cyst walls, embolization of AVM using ONYX, temporotomy). In 33 cases (41.25%), the methods were combined. The choice of surgical treatment strategy was based on individual characteristics of the disease manifestation and course, evaluation of structural and functional disorders using neuroimaging and electrophysiological methods, as well as intraoperative diagnostics and morphometry data. The quality of life before and after the surgery was assessed using the Quality of Life Childhood Epilepsy (QOLCE) questionnaire [21] adapted by Melikyan EG, et al. [13]. Short-term (up to 1 year) and long-term (2 to 7 years) surgery outcomes were analyzed using the Engel Epilepsy Surgery Outcome Scale (Engel J.., 1993). We recorded the patient data necessary for the study in the child’s personal card we have created, and entered these data in a spreadsheet in Microsoft Excel and Microsoft Access 2007 for Windows. Statistical analysis was performed using software systems STATISTICA 10.0 and IBM SPSS 22 for Windows. We used descriptive methods with calculation of absolute and relative frequencies for categorical variables (n, %) and mean magnitudes (mean values, mode and median) and dispersion (minimum and maximum values, standard deviation-SD) for continuous variables. To compare the proportions of categorical variables, we used χ2 Pearson correlations with Bonferroni corrections for multipair comparisons. To compare the Engel Score outcomes and QOLCE results before and after the surgery, as well as for patient groups with Engel Class I and Engel Class II-IV surgery outcomes with their QOLCE results, we used Wilcoxon signed rank test and MannWhitney U test. The analysis results were considered as statistically significant at a significance level p ≤ 0.05.
The time intervals between the surgery date and the collection of data on the long-term outcome were from 1 year to 7 years, with an average of 2.26 ± 0.18 years. Long-term (more than 4 years) surgery outcomes have been studied in 44 (55%) patients. The observation time constituted 1 to 5.2 years. In the long term, the Engel Class I outcome (free of disabling seizures) was achieved in 54.54%, Engel Class II-in 29.54%, Engel Class III-in 11.35%, Engel Class IV- in 4.54% of patients. The first antiepileptic drug administered in the treatment of epilepsy in 1/2 cases was valproates and in 1/4-carbamazepine and oxcarbazepine, the rest were phenobarbital in 7, benzonal in 1, topiramate in 2, diphenine in 1 child. 80% of the children had a history of more than 2 anticonvulsants, and in 30% 4 or more drugs were changed. On admission, 40% of children received treatment with one drug, in other cases, 47.5% took 2, 7.5%-3, and 2.5%-4 antiepileptic drugs. In most cases (56.25%) the basic anticonvulsants were valproates, and in 1/3 observations-carbamazepine. Modern antiepileptic drugs (oxcarbazepine, levetiracetam, lacosamide, topiramate, lamotrigine, zonisamide, fycompa) were used in 60% of cases. In 14 (17.5%) children the dosage of anticonvulsants exceeded the average therapeutic dose, which was confirmed by an increase in the serum of 6 children. Adverse outcome was more common in patients who received 4 or more antiepileptic drugs (20%) when compared to children who received less than 4 anticonvulsants (72.41%) (p<0.01). The complications in the short term were partial CN III paresis in 1 child, transient hemiparesis in 2 and transient contralateral upper quadrant hemianopia in 2 children. No operation mortality was observed. In 2 patients after temporal lobectomy, an extra-temporal resection was required in a year (due to the revealed frontal lobe focal cortical dysplasia), and as a result, an Engel I outcome was achieved. In 1 patient after a temporal resection with a 50% reduction in seizures in the postoperative period and a vagus stimulator implantation the outcome was Engel I. One patient underwent the arachnoid cyst walls excision of the temporal lobe pole, but due to the seizures recurrence, a resection of cystic gliotic lesions in a temporal lobe was performed, and the outcome was Engel II. In 1 patient following the evacuation of the temporal lobe cyst and encephaloduroarteriosynangiosis, in 3 months the temporal lobe was removed due to seizures recurrence. The study of quality of life in patients before and after surgery, using the QOLCE questionnaire, has identified higher values (Table 1) when assessing the Social Interactions, Social Activities, as well as Behavior subscales in children (48.33 ± 10.1, 46.53 ± 6.58 and 46.37 ± 4.61 points, respectively). When comparing the results, the positive effect of surgery on such quality of life subscales as Physical restrictions (+8.82), Attention/concentration (+4.97), Memory (+7.43), Language (+13.21), and QOL (+1.38) was identified. The Energy/Fatigue, Other Cognitive Processes, Social Interactions, Self Esteem and Behavior subscales did not show any significant changes when compared to the preoperative condition. The number of negative values in Anxiety and Depression subscales increased. The postoperative values of Control/Helplessness, Stigma and General Health subscales decreased unexpectedly. In general, the surgery did not adversely affect the children’s quality of life, and even improved it in certain subscales.
| Subscale | Preoperative score mean |
Postoperative score mean |
Mean value variation |
| Physical restrictions | 20.74 ± 5.53 | 29.56 ± 4.52 | 8.82 |
| Energy/fatigue | 45.83 ± 2.95 | 44.53 ± 3.78 | -1.3 |
| Attention/ concentration | 31.11 ± 7.85 | 36.08 ± 5.99 | 4.97 |
| Memory | 37.4 ± 8.96 | 44.83 ± 6.91 | 7.43 |
| Language | 31.4 ± 8.9 | 44.61 ± 6.49 | 13.21 |
| Other cognitive | 35.64 ± 8.59 | 36.97 ± 6.76 | 1.33 |
| Depression | 45.6 ± 4.52 | 41.54 ± 3.14 | -4.06 |
| Anxiety | 43.05 ± 5.6 | 39.09 ± 3.61 | -3.96 |
| Control/helplessness | 55.09 ± 8.2 | 45.57 ± 4.62 | -9.52 |
| Self esteem | 40.97 ± 3.73 | 46.33 ± 4.35 | 5.36 |
| Social interactions | 52.08 ± 8.98 | 48.33 ± 10.1 | -3.75 |
| Social activities | 48.88 ± 2.13 | 46.53 ± 6.58 | -2.35 |
| Stigma | 50 ± 17.08 | 48.08 ± 11.83 | -1.92 |
| Behavior | 46.2 ± 2.66 | 46.37 ± 4.61 | 0.17 |
| General health | 41.67 ± 10.2 | 35.3 ± 3.75 | -6.37 |
| QOL | 41.67 ± 5.89 | 43.05 ± 6.33 | 1.38 |
| Overall QOL | 41.71 ± 3.02 | 40.74 ± 3.39 | -0.97 |
Table 1: Results of assessment of quality of life in children in preoperative and postoperative periods by QOLCE subscales.
The analysis of QOLCE questionnaire results vs. surgery outcomes was performed comparing the outcome groups under Engel Class I (free of disabling seizures) and Engel Classes II-V (rare occurrence of seizures or no significant change in the occurrence of seizures) (Table 2).
| QOLCE subscales | After surgery | |||||
| Engel I | Engel II-IV | |||||
| Mean | Me | St. err. | Mean | Me | St. err. | |
| Physical restrictions | 40,57 | 35,71 | ± 5,92 | 18,55 | 21,87 | ± 4,63 |
| Energy/fatigue | 51,39 | 50 | ± 3,26 | 35,71 | 37,5 | ± 6,35 |
| Attention/ concentration | 43,89 | 55 | ± 8,97 | 24,37 | 19,37 | ± 3,6 |
| Memory | 52,31 | 54,17 | ± 9,85 | 33,61 | 25 | ± 7,69 |
| Language | 53,16 | 56,25 | ± 9,6 | 33,63 | 34,37 | ± 6,82 |
| Other cognitive | 47,21 | 50 | ± 9,92 | 23,8 | 25 | ± 6,41 |
| Depression | 41,67 | 43,75 | ± 4,42 | 41,4 | 42,71 | ± 4,78 |
| Anxiety | 44,44 | 41,67 | ± 4,6 | 33,07 | 30,21 | ± 5,11 |
| Control/ helplessness | 50 | 56,25 | ± 6,42 | 39,87 | 43,75 | ± 6,43 |
| Self esteem | 41,11 | 40 | ± 3,51 | 53,04 | 50 | ± 8,57 |
| Social interactions | 72,61 | 75 | ± 10,72 | 27,08 | 16,65 | ± 12,47 |
| Social activities | 52,68 | 56,25 | ± 8,79 | 41,15 | 45,83 | ± 9,77 |
| Stigma | 70,83 | 75 | ± 13,57 | 28,57 | 0 | ± 15,84 |
| Behavior | 54,44 | 51,47 | ± 6,17 | 37,29 | 41,84 | ± 5,67 |
| General health | 41,67 | 50 | ± 4,17 | 28,12 | 25 | ± 5,67 |
| QOL | 58,33 | 50 | ± 8,33 | 27,78 | 25 | ± 6,51 |
| Overall QOL | 50,03 | 43,44 | ± 4,68 | 31,46 | 31,40 | ± 2,34 |
Table 2: Results of quality of life in children assessment by surgery outcome.
In the long term, when comparing the status of cognitive functions, a significant (p=0,05) improvement of language functions was observed in a patient group with surgery outcome Engel Class I. In contrast to the results of Classes II-IV, a significant improvement of concentration/attention, memory and other cognitive processes was also observed in the first group (Figure 1).

Figure 1: Outcome-specific distribution of post-surgical patients according to QOLCE questionnaire in Cognitive Function subscale.
With complete freedom from disabling seizures, the level of physical restrictions was sharply reduced, and parents noted the children’s energy and a positive effect on their behavior. In Energy/Fatigue and Physical Restrictions subscales these changes were statistically significant (p<0,05) (Figure 2).
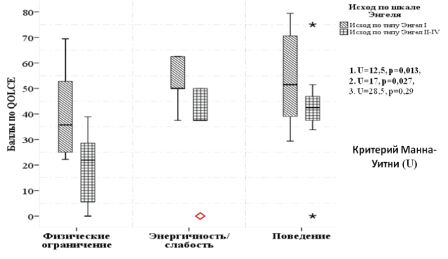
Figure 2: Outcome-specific distribution of post-surgical patients according to QOLCE questionnaire in Physical restrictions, Energy/ Fatigue and Behavior subscales.
In patients with Engel Class I outcome, a significant improvement of QOL and Overall QOL subscales (p<0,05) was also identified (Figure 3). No significant impact on the General Health subscale was noted.
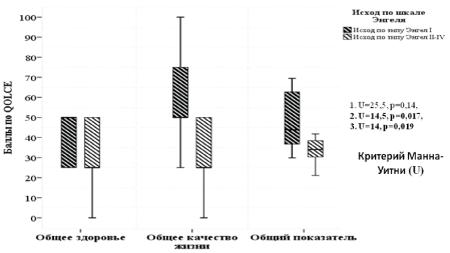
Figure 3: Outcome-specific distribution of post-surgical patients according to QOLCE questionnaire in General Health, QOL and Overall QOL subscales.
The surgery outcomes with respect to their effect on seizure frequency, assessed by Engel Epilepsy Surgery Outcome Scale, showed that freedom from disabling seizures in the long term (3 to 7 years) has been achieved in 54.54% of patients. This value fits into the percentage of favorable outcomes obtained by other researchers [24,25].
When comparing the children’s quality of life in preoperative vs. postoperative periods, the positive effect of surgery on such subscales as Social Interactions and Activities, Behavior, Physical Restrictions, Concentration/Attention, Memory and Language has been identified. In general, a neutral or positive effect of treatment on most of the quality of life subscales was observed. A significant (p<0.05) positive change in quality of life indicators has been proved at a favorable outcome corresponding to Engel Class I. Such changes took place in Language, Physical Restrictions, Energy/Fatigue, QOL and Overall QOL subscales. The data obtained correspond to the results of other authors who also demonstrated improvement in speech functions, energy and physical functioning, behavior, self-esteem, emotional functioning and general health [7]. In most cases, we did not change the treatment with antiepileptic drugs after the operation, but in some cases, the dose was adjusted. One would expect some reduction in the number of drugs used and their dosages, and these changes could contribute to postoperative benefits in some of the parameters studied. To generate high-strength recommendations, further multi-center prospective studies on large populations of patients are necessary, with a detailed analysis of the relationship between factors that influence surgery outcome and the child’s psychosocial well-being. To more accurately assess the change in health indicators and the quality of life of patients before and after surgery, standardization of the algorithm for preoperative examination of patients and the use of equivalent psychometric tools are also required.
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Abramov KB, Sarsembayeva DA, Ivanov AY, Odintsova GV, Khachatryan WA, et al. (2018) Changes in Quality of Life in Children after Surgical Treatment of Drug-Resistant Temporal Lobe Epilepsy. J Neurol Neurobiol 4(2): dx.doi.org/10.16966/2379-7150.149
Copyright: © 2018 Abramov KB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access