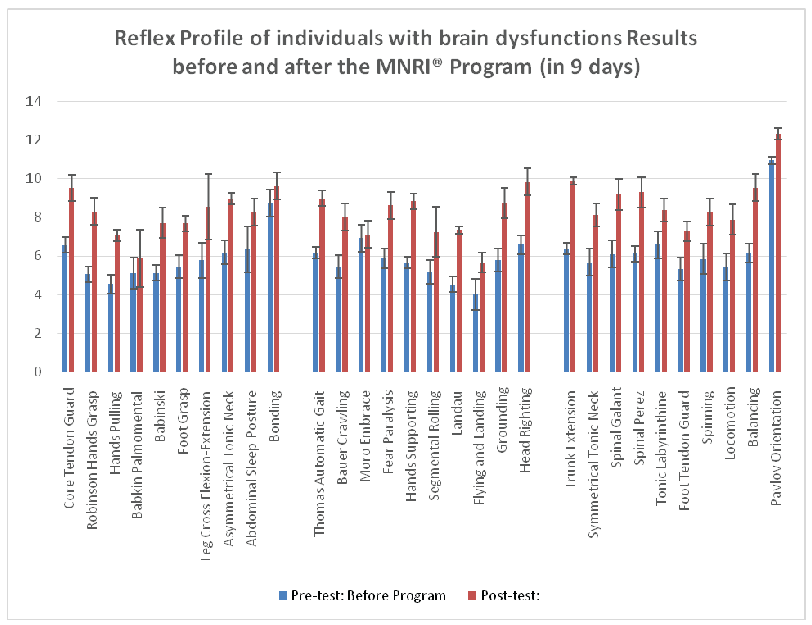
Figure 1: Dynamic of changes in reflex patterns before and after the MNRI® Program of individuals with brain dysfunctions (n=53).


J Lucas Koberda1* Nelli Akhmatova2 Elina Akhmatova2 Andrew Bienkiewicz3 Katarzyna Nowak4 Halina Nawrocka5
1Tallahassee NeuroBalance Center, Tallahassee, Florida, USA*Corresponding author: J Lucas Koberda, CEO of Brain Enhancement Inc. and Director of Tallahassee NeuroBalance Center, 4838 Kerry Forest Parkway, Tallahassee, Florida 32309, USA, Tel: 850-459-8263; E-mail: JLKoberda@yahoo.com
Masgutova Neurosensorimotor Reflex Integration MNRI® is a non-invasive method for evaluating and improving neurodevelopmental delays in children having neurological dysfunctions such as cerebral palsy (CP), Autistic Spectrum Disorders (ASD), and other types of neurological dysfunctions. Changes in the reflex patterns of 53 individuals with CP and other types of brain damage were used as objective measures. They were evaluated before and after participation in an intensive MNRI® rehabilitation treatment for 8 days 6 hours daily. Two evaluation tools were used for comparative analysis of the MNRI® therapy results-brain mapping (also called quantitative EEG) and MNRI® Reflex Assessment. Both of these evaluation methods showed substantial improvements-positive changes in brain mapping after therapy completion as well as a major clinical improvement. Assessments taken after completion of the rehabilitation program showed significant improvement in the children’s reflex functions. This data indicates that a neurodevelopment and overall functioning of individuals with CP and other conditions tested is not static and could be successfully improved with this novel form of therapy.
MNRI; CP; ASD; Neuromodulation; EEG; QEEG; Brain mapping; Sensory-motor reflex
Childhood cerebral palsy (CP, paralysis cerebralis infantum) or Little’s disease is a syndrome of various disorders of the motor-posture system resulting from permanent, non-progressive brain damage in the early stages of brain development [1-5]. Childhood cerebral palsy disorders result from damage to the developing brain during: pregnancy (20%), the perinatal stage (60%), or first years of life (20%) [6]. Causes of CP include: abdominal injuries to the mother, chronic disease during pregnancy, malformations, fetal hypoxia, infection, the influence of ionizing radiation, drugs or toxins including cigarette smoking and alcohol consumption, perinatal trauma, prematurity, brain injury, hypoxia after birth, severe neonatal jaundice, and neuro-infections [2-9].
There are large numbers of individuals diagnosed with CP in the world ranging from 1.5 to more than 4 per 1,000 live births. In the USA about 10,000 infants are diagnosed with CP each year [10]. The United Cerebral Palsy Association reports that more than 764,000 Americans have CP. Each year preschools have up to 1,500 students with CP. Most of the children (77.4%) with CP have spastic or pathological muscle hypertension. They also can have at least one co-occurring condition 35% to 50% of children with CP have seizure disorders and some level of mental retardation, learning disabilities, vision, speech, and hearing problems. A few (6.9%) have co-occurring ASD. Over 40-45% of the children identified with CP has affected motor coordination and are limited in their ability crawl, walk independently, run, or play [11]. Another study shows that 31% of children with CP need to use special equipment such as walkers or wheelchairs [12].
Depending on the area of damage in the central nervous system and its symptoms, four main forms of this disease are distinguished (according to the international classification system Gross Motor Function Classification System-GMFCS): spastic (pyramidal; 70-80% of all CP cases; hypertonia, or increased muscle tone–muscles and their movements are stiff and jerky), dyskinetic (extrapyramidal, 15% of CP cases; lesions in the basal ganglia as result of bilirubin encephalopathy or/and hypoxic-ischemic brain injury), ataxic (cerebellum/brain stem; 5% of CP cases poor motor coordination, balance and muscle tone regulation; tremors, with possible speech and oral problems), and mixed (spastic and non-spastic) forms which are the most numerous [1,2,8,13].
Brain damage is an injury causing the destruction or deterioration of brain cells occurring after birth and is a non-degenerative neurological disease [4,14]. The Brain Injury Association of America reports that every year about 2.6 million people receive a brain injury resulting from trauma, tumor, stoke, or other illnesses. More than 5 million Americans suffering traumatic brain injury require assistance in performing daily activities [15].
There are two types of brain injury-a traumatic brain injury (TBI), caused by an external force (damage to brain cells such as from a concussion, wound to brain-bullet or diffuse axonal injury), and an acquired brain injury that occurs at the cellular level (associated with pressure on the brain by a tumor or stroke, etc.) which disrupts normal functioning of the brain [16-18].
There is also brain damage that results from genetic sources or birth trauma called congenital brain damage (though not defined as brain damage in standard diagnosis).
The scope of methods addressing the above diseases and problems that these dysfunctions cause are numerous from traditional forms of medical support (medications, surgical procedures, orthotics, etc.), PT (physiotherapy), OT (occupational therapy), SP (speech therapy), cognitive-behavioral therapy, neurofeedback [19], massage therapy, hydrotherapy, dietary advice, and other alternative treatments including NDT Bobath, Doman-Delacato, Vojta, [20-22], the Wroclaw Improvement System [23] and many others.
A relatively new method, MNRI® described in various works [24-30] addresses the neurosensorimotor aspect of early sensory-motor patterns and reflexes to support neurodevelopment and motor-static (postural) issues of children and adults with CP or traumatic brain injury and ABI.
The demand for neurorehabilitation of children with impaired sensorimotor functions due to damaged central nervous systems has become greater than in the past because of two-primary reasons: the number of children with this disorder is high and increases year by year [11]. In addition, there is an emergence of more promising therapy programs available that address improving functions for individuals with neurological deficits [4]. In this study the Masgutova Neurosensorimotor Reflex Integration MNRI® technique was used as therapy for patients with cerebral palsy and other types of brain damage including stroke, anoxic brain injury as well as autistic spectrum disorders. One of the objectives of this paper is a demonstration of the effectiveness of the MNRI® therapy. Brain mapping (QEEG) and the MNRI® Reflex Development Assessment was used as objective means. Prior research with brain mapping (QEEG) for measuring the MNRI® therapy effects has been done previously [27]. This article further advances this topic and addresses the effectiveness of MNRI® therapy using the tools of QEEG and Reflex Assessment. To show this, we have investigated the hypothesis that parameters of the MNRI® Reflex Assessment will correlate with the parameters of brain mapping, measured before and after the Reflex Integration treatment session, and will show the enhancement of neurodevelopment in the individuals with CP and other forms of brain dysfunction. This report presents the results of 53 patients who were diagnosed with different neurological conditions and subjected to MNRI® therapy.
Assessments of 30 reflex patterns (coded X1-X30) of individuals in the study group were done twice: prior the MNRI® therapy and after its completion of eight days at the training conference.
Evaluation of the reflex patterns was provided based on the neurophysiological definition of the inborn reflex and its parameters such as: sensory-motor coordination, direction of the response, intensity (muscle tone regulation), timing/dynamics of the response, symmetry [27,31]. Every parameter is tested according to four determined features. For example, assessment of the first parameter of ‘sensory-motor coordination’ (sensory stimulus and bio-mechanical aspect of the motor response) in the Hands Grasp Reflex tested the next four features: 1) tactile stimulation on the base of a closed palm in a more intense proprioceptive way-deeper to activate palm flexors, should trigger a stronger grasp response, 2) all fingers are closed and thumb is between index and middle fingers, 3) elbow can be easily extended in front of the body for 180 degrees, and shoulder for 90 or more degrees (differentiation between elbow and palm and shoulder is evident/in norm), 4) arm/palms are directed horizontally to the ground (no abnormal abduction/adduction in the wrist joint). Every parameter has its own four described features.
The assessed data was analyzed comparative to the child’s age, neurological norms and abnormality features. Scoring of a reflex pattern within the parameters above were assigned on a scale of 0-4, with 4 indicating full display of all four features in a parameter, and 0 indicating that normal/correct responses or features in a certain parameter were absent. The maximum score for 5 parameters and 4 features in each reflex pattern gave a total of 20 points-the highest level of maturity or integration (Table 1). A summary of scores are: between 11 to 20 represent functional development, and below 10 points (0-9)-dysfunctional or abnormal development. The scores of 10 to 11.99 are marginal results between functional and dysfunctional states of reflex patterns. Scores of 16-17.99 represent the norm. The scoring system has been validated by statistical research carried out by math Professor A. Krefft [27,32], and also the ANOVA test (IBM SPSS Statistics Grad Pack 22.00); results were considered statistically significant with p values (M ± SD) less than 0.001 and interpreted as significant, and not significant at p>0.05.
Normal Function |
Dysfunction/Pathology |
||
Points |
Level of reflex integration |
Points |
Level of reflex |
20 |
Full/Complete integration |
10-11.99 |
Marginal pathology and dysfunction |
18-19.99 |
Mature and integrated |
8-9.99 |
Incorrect, light |
16-17.99 |
Correctly developed-normal |
6-7.99 |
Dysfunction |
14-15.99 |
Functional, but low level of |
4-5.99 |
Severe dysfunction |
12-13.99 |
Functional, but very low |
2-3.99 |
Pathology |
10-11.99 |
Marginal pathology and dysfunction |
0-1.99 |
Severe pathology |
Table 1: Clinical evaluation: criteria for Reflex Assessment scores (in points 0-20).
Reflex patterns were further categorized for convenience according to body movement planes, with ten patterns in each, corresponding to sagittal (medial-lateral), horizontal (superior-inferior), and dorsal (anterior-posterior) body movement planes [28,31,33].
Additionally the evaluation of changes in abilities and everyday functioning was administered using the Questionnaire of Dynamic Changes in Children’s Functioning [34] within next 10 main areas: 1) Sensory-motor integration; 2) Behavior regulation and self-protection; 3) Emotional regulation; 4) Self-awareness; 5) Communication/interaction; 6) Stress vulnerability/resilience; 7) Physical health; 8) School skills (reading comprehension, writing, math, etc.); 9) Cognitive processes (attention, memory, thinking, language) and learning; and 10) Motivation for achievement and learning. The scoring system ranging the results from ‘0’ to ‘20’ points, with ‘0’ and ‘1’was denoting the lowest-developed features and ‘20’ indicating normal and very well developed. Criteria given for evaluation of every area were such as: ‘norm’ in function/ability, ‘close to norm,’ ‘displays some difficulties,’ ‘displays major difficulties,’ or ‘pathological’. Statistical analysis was done using the ANOVA test (IBM SPSS Statistics Grad Pack 22.00 and the Mann-Whitney U-test, using Statistica (version 6.0; Stat Soft Inc., Tulsa, OK, USA); results were considered statistically significant with p values (M ± SD) less than 0.05 interpreted as significant, and not significant at p>0.05.
All specialists leading the evaluations were certified by the NIH (National Institute of Health, Office of Extra mutual Research) “Protecting Human Research Participants” in 2012-2015.All participants were assigned codes to protect anonymity. Receipt of informed consent was received from all participants’ parent or legal guardian. MNRI® Assessments were conducted and treatment administered by designated Specialists or MNRI® Core Specialists who have successfully completed the requirements for Continuing Professional Education in MNRI® and clinical hours (www.MasgutovaMethod.com).
MNRI® was first developed in 1989 in Russia and then further in Eastern Europe to treat individuals with certain types of sensorimotor or reflex development deficits, behavior disorders, disorders of speech or language development, and learning disabilities. It was introduced to the USA in 1996 and has since then gradually become adopted in many other countries. Our clinical observations have shown that MNRI® facilitates the neurodevelopment in individuals having various neurological deficits and seems to enable them to reroute and improve their early movements, reflexes, coordination systems, and skills to enable more optimal functioning, development, and learning [27-29]. The MNRI® therapy program is based on the supposition that impaired reflex circuits can be reconstructed. This retraining of reflexes appears to result in the awakening of genetic sensorimotor memory in individuals with CP and brain damage- including many severe cases. Our observation has been that significant positive changes occur in physical strength, immune activity, and improvement in cognitive, emotional, social, and motor abilities [26-28,30].
MNRI® is based on exercises and techniques called ‘repatterning’, which means reeducation, recoding, rerouting the reflex nerve pathways specific for dynamic and postural reflex patterns (e.g., Babinski, Automatic Gait, Bauer Crawling, Hands Grasp, and others). The stimulation of those reflex pathways is aimed at strengthening and stabilizing traces of genetic sensory-motor memory and the activation of innate defensive mechanisms of the body’s brain ‘alarm’ system (HPA stress axis or ‘hypothalamus->pituitary gland->adrenals’ cycle activation described by H. Selye [35], in times of stress or danger. MNRI® exercises stimulate innate neuro-regulation mechanisms and resilience in the stress and immune systems [30,33]. Repatterning activates the extrapyramidal nerve system (peripheral nerves, spinal cord, brain stem, diencephalon) responsible for automatic mechanisms and processes, the extension of links between neurons, the growth of neural nets, myelination, and the creation of new nerve routing, as described by Sechenov, Anokhin, Haines, Virella et al. [36-39].
Fifty-three patients (age 2-47 years) diagnosed mainly with ASD, CP, Traumatic Brain Injury (TBI) and other neurological dysfunctions underwent the MNRI® repatterning exercises at MNRI® Family Conferences for a specific treatment period. The program involved a typical MNRI® therapeutic process which included eight consecutive progressive sub-programs chosen in such a way as to affect various parts of the body to activate its sensory-motor functions. The MNRI® process included the following MNRI® sub-programs: Reflex Repatterning, Proprioceptive-Cognitive Integration, Neuro-Structural Reflex Repatterning, Neuro-Tactile Integration, Breathing Reflex Integration, Oral-Motor/VisualAuditory Reflexes Integration, Archetype Movements Integration, and Stress and Traumatic Stress release. Each session lasted 50 minutes with a 10 minute break between three sessions in the morning, 1.5 hour lunch break and 3 sessions after lunch followed by group lectures and training for caregivers in the evening. The training session lasted 8 days (4 days of work with 6 hours daily, one day off, and another 4 days of work with 6 hours daily). The training was provided by MNRI® certified specialists.
Deymed Truscan 32 (Deymed Diagnostic, Payette, ID) EEG equipment was combined with Neuroguide QEEG (Applied Neuroscience, Inc.) software. QEEG analysis was completed using commercially available Neuroguide software (Applied Neuroscience, Inc.) and previously recorded 19 channel digital EEG (Deymed Diagnostic, Payette, ID).
Approximately 1-3 minutes of artifact-free eyes closed or eyes open EEG segments were selected after previously recording EEG with the Deymed, Truscan 32, (Deymed Diagnostic, Payette, ID) and subjected to further QEEG analysis.
For evaluation, two EEG sessions were completed-before and after the MNRI® treatment program of six hours a day for eight days. Spontaneous EEG activity was recorded in a 10-20 electrode system using 16 leads. The EEG recording underwent an elimination of artifacts, after which the software produced QEEG maps of the EEG frequencies. The fast Fourier transform (FFT) algorithms were used in the computer analysis. Spectral maps were compared in relative and absolute scale for the measured signals obtained before and after MNRI® rehabilitation. We analyzed the EEG spectrum from 0.0 Hz to 30Hz, divided into five sub-ranges: 0.5-4.0 Hz (delta), 4.0-8.0 Hz (theta), 8.0-12.0 Hz (alpha), 12.0-20 Hz (beta) and 20-30 Hz (high beta).
The results of the MNRI® Assessment of reflex patterns in the Study Group of individuals with CP and other neurological conditions.
Table 2 demonstrates the Diagnostic Quality Features (X1 -X30) within body movement planes (S=sagittal; H=horizontal; D=dorsal), levels of reflex development, and Assessments results before and after participation in MNRI® integration therapy in the Study Group. Note: ‘*’ equals the statistical significance at p>0.001.
Diagnostic Quality/ Feature |
Body Movement Plane |
Reflex |
Reflex Profile of individuals with brain dysfunctions results before and after the MNRI® Program (in 9 days) |
||
Study Group |
|||||
|
(n=53) |
||||
|
|
|
Pre-test: |
Post-test: |
|
X 1 |
S |
Core Tendon Guard |
6.57 ± 0.4 |
9.53 ± 0.7* |
|
X2 |
S |
Robinson Hands Grasp |
5.05 ± 0.4 |
8.31 ± 0.7* |
|
X 3 |
S |
Hands Pulling |
4.54 ± 0.5 |
7.07 ± 0.3* |
|
X 4 |
S |
Babkin Palmomental |
5.11 ± 0.8 |
5.87 ± 1.5 |
|
X5 |
S |
Babinski |
5.12 ± 0.4 |
7.72 ± 0.8* |
|
X6 |
S |
Foot Grasp |
5.45 ± 0.6 |
7.69 ± 0.4* |
|
X7 |
S |
Leg Cross Flexion-Extension |
5.77 ± 0.9 |
8.54 ± 1.7* |
|
X8 |
S |
Asymmetrical Tonic Neck |
6.19 ± 0.6 |
8.98 ± 0.3* |
|
X9 |
S |
Abdominal Sleep Posture |
6.35 ± 1.2 |
8.26 ± 0.7 |
|
X10 |
S |
Bonding |
8.76 ± 0.7 |
9.63 ± 0.7 |
|
X11 |
H |
Thomas Automatic Gait |
5.87 ± 0.5 |
8.62 ± 0.7* |
|
X12 |
H |
Bauer Crawling |
5.66 ± 0.3 |
8.83 ± 0.4* |
|
X13 |
H |
Moro Embrace |
5.17 ± 0.6 |
7.24 ± 1.3 |
|
X14 |
H |
Fear Paralysis |
4.52 ± 0.4 |
7.35 ± 0.2* |
|
X15 |
H |
Hands Supporting |
4.01 ± 0.8 |
5.66 ± 0.5 |
|
X16 |
H |
Segmental Rolling |
5.78 ± 0.6 |
8.74 ± 0.8* |
|
X17 |
H |
Landau |
6.58 ± 0.5 |
9.84 ± 0.7* |
|
X18 |
H |
Flying and Landing |
5.87 ± 0.5 |
8.62 ± 0.7* |
|
X19 |
H |
Grounding |
5.66 ± 0.3 |
8.83 ± 0.4* |
|
X20 |
H |
Head Righting |
5.17 ± 0.6 |
7.24 ± 1.3 |
|
X21 |
D |
Trunk Extension |
6.38 ± 0.3 |
9.88 ± 0.2* |
|
X22 |
D |
Symmetrical Tonic Neck |
5.68 ± 0.7 |
8.13 ± 0.6* |
|
X23 |
D |
Spinal Galant |
6.1 ± 0.7 |
9.19 ± 0.8* |
|
X24 |
D |
Spinal Perez |
6.12 ± 0.4 |
9.3 ± 0.8* |
|
X25 |
D |
Tonic Labyrinthine |
6.59 ± 0.7 |
8.37 ± 0.6* |
|
X26 |
D |
Foot Tendon Guard |
5.31 ± 0.6 |
7.27 ± 0.5* |
|
X27 |
D |
Spinning |
5.85 ± 0.8 |
8.29 ± 0.7 |
|
X28 |
D |
Locomotion |
5.44 ± 0.7 |
7.89 ± 0.8 |
|
X29 |
D |
Balancing |
6.14 ± 0.5 |
9.54 ± 0.7* |
|
X30 |
D |
Pavlov Orientation |
10.96 ± 0.2 |
12.34 ± 0.3* |
|
|
|
|
|
||
%/Patterns |
Severe dysfunction (4-5.99 |
56.7%/17 |
6.7%/2 |
||
%/Patterns |
Dysfunctional (6-7.99 |
36.7%/11 |
26.7%/8 |
||
%/Patterns |
Light Dysfunction (8-9.99 |
3.3%/1 |
63.3%/19 |
||
%//Patterns |
Marginal between dysfunctional and functional state (10-11.99) |
3.3%/1 |
0 |
||
%//Patterns |
Functional but very low level of development (12-13.99) |
0 |
3.3%/1 |
||
%//Patterns |
Functional but low level of development (14-15.99) |
0 |
0 |
||
%//Patterns |
In norm (16-17.99) |
0 |
0 |
||
Table 2: Reflex Assessment results before and after the MNRI® program of individuals with brain dysfunctions (n=53).
First, the level of development of reflex patterns in children with brain developmental deficits/disorders was overall immature and dysfunctional, particularly: 17 patterns-‘severe dysfunctional’ level (4-5.99 points), 11 patterns-‘dysfunctional’ (6-7.99 points), 1 pattern-‘light dysfunctional’ (8-9.99 points), and 1 pattern-‘marginal between dysfunctional and functional’ level (10-11.99) (Figure 1).

Figure 1: Dynamic of changes in reflex patterns before and after the MNRI® Program of individuals with brain dysfunctions (n=53).
Second, the results of post-assessment after the MNRI® treatment in Study Group (children with neurological deficits) show that 22 of their reflex patterns (73.3% out of 30 patterns) have changed within 9 days of the MNRI®. This data demonstrates that dysfunctional or pathological reflexes are not static and can be changed with specially oriented manual neuromodulation treatment vs traditional concepts approaching the abnormality as a non-changeable state. The data shows increase of 15/50% of reflex patterns to the next level from ‘severe dysfunctional’ (in preassessment 17/56.7% patterns) to “dysfunctional” (27/90%) and 18/60% patterns to ‘light dysfunction’ level (Figure 2).
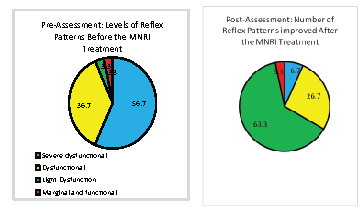
Figure 2: Changes in level of reflex functions before and after the MNRI® Program of individuals with brain dysfunctions (n=53).
Third, results of the post-assessment demonstrating significant improvement in reflex patterns still show that they are on a dysfunctional level and need further regular work. Our long-term clinic experience shows the need of 2-3 years of work in the Program to provide the development of reflex patterns to a functional level.
Additional analysis of the average values and standard deviations was carried out for three synthetic variables: ZS (sagittal body plane), ZH (horizontal), and ZD (dorsal) and ZC (cumulative) in pre-and post-tests levels of reflex pattern development in the Study Group and Control Group. The reflex patterns development results in each body plane are statistically significant only in the Study Group that participated in MNRI® intervention (Table 3).
The results in Table 3 were validated statistically using data on a level of synthesized function Z=f(x) [31] as well as the nonparametric comparison of two variables by Wilcoxon Matched Pairs test (p>0.001; Statistical Program). The statistical evaluation of the data demonstrates significant change in all synthesized variables (with 10 reflex patterns in each) and a high degree of effectiveness for the MNRI® program.
Average values and standard deviations for three synthetic variables: ZS (sagittal body plane), ZH (horizontal), and ZD (dorsal) |
||||||
Groups |
Variables |
Before and after the MNRI® intervention |
|
|||
Before |
After |
ANOVA |
||||
Mean |
S.D. |
Mean |
S.D. |
p < |
||
Study Group |
ZC |
0.4124 |
0.0218 |
0.6056 |
0.0178 |
0.001 |
ZS |
0.4072 |
0.0198 |
0.5815 |
0.0193 |
0.001 |
|
ZH |
0.3968 |
0.0188 |
0.5987 |
0.0176 |
0.001 |
|
ZD |
0.4137 |
0.0205 |
0.5918 |
0.0195 |
0.001 |
|
|
|
|
|
|
|
|
Control Group (n=50) Neurotypical development |
ZC |
0.2812 |
0.1884 |
0.3416 |
0.1722 |
0.05 |
ZS |
0.2988 |
0.1676 |
0.2961 |
0.1674 |
0.05 |
|
ZH |
0.2861 |
0.1583 |
0.2554 |
0.1592 |
0.05 |
|
ZD |
0.3087 |
0.1275 |
0.3421 |
0.1427 |
0.05 |
|
Table 3: Results of comparative analysis of average values and standard deviations with three synthetic variables: ZS (sagittal body plane), ZH (horizontal), and ZD (dorsal) and ZC (cumulative) in pre- and post-tests levels of reflex patterns development in the Study Group and Control Groups 1 and 2.
Out of 53 patients subjected to MNRI® therapy 42 (79%) showed positive changes in previously identified QEEG abnormalities. Most of patients involved in this study were diagnosed with ASD (n=22) and CP (n=19), however other diagnoses were also represented including ADHD (n=4) traumatic or anoxic brain injury (n=4), prior stroke (n=2) dystonia (n=1) and PTSD (n=1).
The most common abnormalities identified on brain mapping before MNRI® therapy was frontal or temporal overexpression of delta or theta power. However, an elevated frontal or occipital beta or high beta power were also frequently recorded. The degree of an improvement after the therapy varied from patient to patient and only a small proportion of patients showed no evidence of brain mapping improvement (n=11/21%). Two representative cases are presented which illustrate typical QEEG response to MNRI® therapy.
Represents 11 year old patient diagnosed with CP. Brain maps showed marked overexpression of frontal delta, theta and beta and high beta power (Figure 3A). In addition, multiple abnormalities in amplitude asymmetry, coherence and phase lag were noted. As we know from our prior papers an elevated frontal delta, theta power as well as increased beta power are frequently seen in individuals with brain dysfunction seen in ASD, ADHD as well as other neurological conditions [40-42].
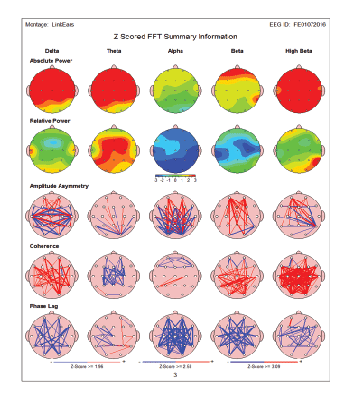
Figure 3A: An11 year old patient diagnosed with CP. Brain maps before MNRI® therapy showed marked over expression of frontal delta, theta and beta and high beta power. In addition, multiple abnormalities in amplitude asymmetry, coherence and phase lag were noted.
After completion of MNRI® therapy there was marked improvement of previously identified elevated beta and high beta power (Figure 3B). In addition, some improvement in coherence (hyper-coherence) was also noted. Similar degree of brain maps improvements were seen in patients after completion of neurofeedback therapy which was reported in our previous publications [41-43].
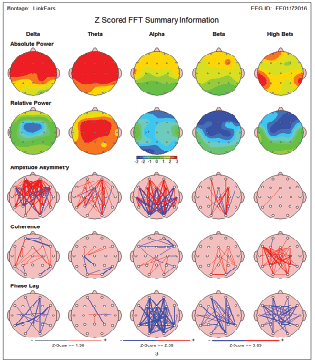
Figure 3B: 11 year old patient diagnosed with CP. QEEG completed after MNRI® therapy revealed marked improvement of previously identified elevated beta and high beta power. In addition, some improvement in coherence (hyper-coherence) was also noted.
This patient QEEG clinical response correlated well with a clinical response (clinical response was statistically significant-A).
Results of Reflex Assessment of this patient shows significant improvement: average level of reflex development from 7.1 points moved to 9.3 (high level of improvement-A). Additional analysis of the changes in abilities show the same positive results-patient moved from 6.7 points before administrating the Questionnaire of Dynamic changes and 9.4 points after (very good improvement- level A).
It is represented by 7 year old patient who was diagnosed with ASD/ ADHD. Brain maps before MNRI® therapy showed evidence of a major increase in frontal delta, alpha as well as frontal and central beta and high beta power (Figure 4A). After completion of MNRI® therapy QEEG maps showed a major improvement of frontal delta and alpha abnormalities. In addition, a marked correction of frontal and central overexpression of beta and high beta power was recorded. This patient QEEG clinical response correlated well with improvement in reflex functions and abilities level (clinical response A). Analysis of Reflex Assessment of the patient demonstrates significant changes: average level of reflex development from 8.6 points moved to 10.5 (high level of improvement - A). Additional analysis of the changes in abilities also shows at positive results-patient moved from 7.6 points before administrating the Questionnaire of Dynamic Changes and 10.7 points after (very good improvement- level A) (Figure 4B).
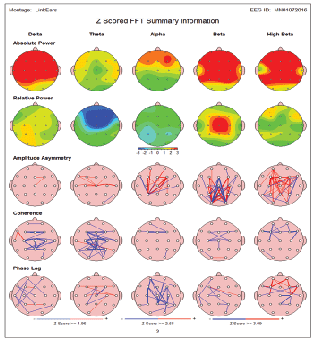
Figure 4A: Case 2-7 year old patient diagnosed with ASD/ADHD. Brain maps before MNRI® therapy showed evidence of a major increase in frontal delta, alpha as well as frontal and central beta and high beta power.
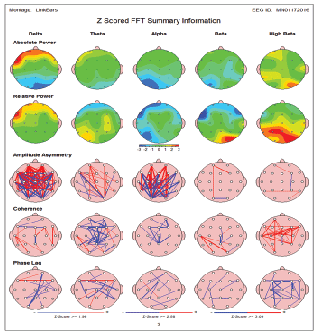
Figure 4B: Case 2-7 year old patient diagnosed with ASD/ADHD. After completion of MNRI® therapy QEEG maps showed a major improvement of frontal delta and alpha abnormalities. In addition a marked correction of frontal and central over expression of beta and high beta power was recorded.
QEEG has been shown to be a very useful tool in evaluation of patients with TBI [44], epilepsy [45], stroke [46], dementia [46], ASD [40] and variety of other neurological conditions [47].
Our current brain mapping study shows and allows us to conclude that rehabilitation using MNRI® in individuals with CP, ASD and other types of neurological dysfunctions causes a reorganization and at least partial normalization of spontaneous brain electrical activity. This was most frequently observed in delta and theta frequency as well as in beta and high beta overexpressed activity. This normalization of QEEG activity is likely correlated with the therapeutic effect of the MNRI® program. This has been confirmed by positive clinical responses that were noted after MNRI® therapy. Our observations were consistent with the findings of other authors who have noticed changes in EEG mapping in children with CP during experiments related to the motor system [48].
Clinical observation by professionals (OTs, PTs, SPs, special educators, and psychologists among others) as well as parents shows that after the MNRI® program patients with CP and other types of neurological dysfunction improved their balance, postural control, motor programming and planning [34]. This therapy also facilitated their coordination of movements, strength, precision, space-time orientation, speed of perception and response, better ‘presence’ of mind, easier focusing, better memorizing, and improvement in language development (receptive and expressive).
This study of the MNRI® effects in repatterning and sensory-motor integration in individuals with CP, ASD showed that their reflex functions improved significantly. This serves as an indication that the neurosensory development and overall functioning of these children are not static and can be improved independently of the neurological pathologies they have. Thus, the MNRI® program offers an effective neuromodulator means of improving the overall functioning of children with CP and brain damage, particularly, reflected in improvement of various functions and abilities in different areas: sensory-motor integration, behavior and emotional regulation, communication; stress resilience; physical health; school skills and achievement motivation. This Program potentially serves as an exemplary tool for children with other neurological deficits and learning disabilities.
This study also shows that changes in affected reflex patterns follow after specific MNRI® therapy intervention and that the improvements in affected reflex patterns do not happen spontaneously in children that have incorrect working reflex patterns. Furthermore, this study demonstrates the importance of corrective intervention therapies that target reflex patterns, the specific units of nervous system functioning. The authors of this article find, based on long-term clinical observations and the collection of scientific data, that the MNRI® program should serve as a basic preliminary therapy, prior to other types of therapy modalities such as physiotherapy, occupational therapy, speech pathology therapy, or sensory integration to help pave the way for their success [49].
Our future research will offer technical information concerning the neurophysiological mechanism and biomechanical aspects of a reflex pattern that MNRI® is able to activate though corrective tools using the reflex pattern as the ‘model scheme’ to improve the reflex circuit parameters and sensory-motor pattern functions in children with neurological deficits. There are particular methods to target the neurosensory-motor components of a reflex pattern and other automatic functions in order to support the maturation and strengthen the lower motor neuron functions and subcortical structures of the brain. This, in turn, allows support of higher executive functions such as postural control, motor coordination, regulation of behavior and emotions, cognitive processes (comparison, analysis, comprehension, language), and personality development as has been proven in work with 53 individuals with brain neurological deficits.
Download Provisional PDF Here
Article Type: Research Article
Citation: Koberda JL, Akhmatova N, Akhmatova E, Bienkiewicz A, Nowak K, et al. (2016) Masgutova Neurosensorimotor Reflex Integration (MNRI) Neuromodulation Technique induces Positive Brain Maps (QEEG) Changes. J Neurol Neurobiol 2(4): doi http://dx.doi.org/10.16966/2379-7150.130
Copyright: © 2016 Koberda JL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access