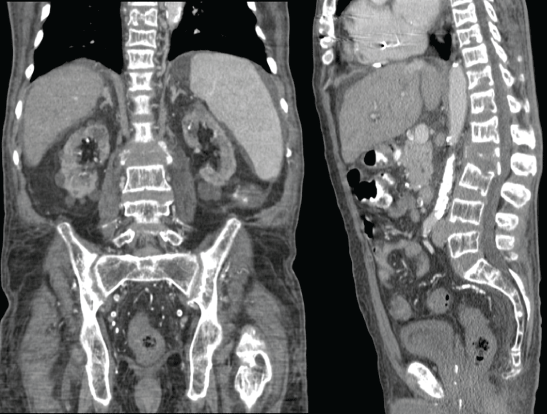
Figure 1: CT contrast of the dorsal and lumbar spine: showing multiple lytic lesions at various levels, gross destruction of the L2 body.


Samira Chandra1 Kriti Devkota2 Sylva Bem2 Cristopher Curtiss2 Amar Swarnkar2 Apurv Khanna2 Manisha Singh1*
1University of Arkansas for Medical Sciences, Arkansas, USA*Corresponding author: Manisha Singh, Division of Nephrology, University of Arkansas for Medical Sciences, Arkansas, USA, E-mail: MSingh@uams.edu
High bone turnover in renal osteodystrophy is a rare clinical entity nowadays due to the early diagnosis and treatment of secondary hyperparathyroidism. Since it is a diagnosis of exclusion, a formidable diagnostic challenge exists when the likelihood of malignancy is high in elderly patients suffering from chronic kidney disease.
In this case report, we describe the clinical picture of such a type of patient. Our patient was an elderly ESRD (End-Stage Renal Disease) patient who presented with a pathological fracture of the spine and pancytopenia. Imaging was highly suspicious for metastatic malignancy. Lytic bone lesion biopsy revealed extensive bone marrow fibrosis indicating the possibility of multiple myeloma. Biopsy from the deposit over symphysis pubis revealed fibrovascular granulation tissue with giant cells. Thorough workup ruled out metastasis and myeloma. Bone marrow biopsy again revealed extensive peri-trabecular fibrosis. Retrospective analysis of other clinical and histological findings hinted toward the downstream effect of hyperparathyroidism.
Serum PTH (Parathormone) was found to be significantly high with ultrasound evidence of parathyroid hyperplasia. The patient’s condition improved after total parathyroidectomy. This case report illustrates the importance of considering secondary hyperparathyroidism as the possibility of a lytic bony lesion, particularly in the CKD (Chronic Kidney Disease) population. High bone turnover in hyperparathyroidism can manifest as osteitis fibrosa cystica (lytic lesion), brown tumor (both lytic lesions and expansile mass), and/or peri-trabecular bone marrow fibrosis. Clinical and histopathological co-relation is crucial for such a diagnosis.
A 64-year-old Black American man presented to the emergency department following a fall from the wheelchair. The patient reported generalized weakness, fatigue, and weight loss over a few months. Past medical history was significant for hypertension, diabetes type2, idiopathic cardiomyopathy with left ventricular hypertrophy post Implantable Cardioverter-Defibrillator (ICD) placement, and ESRD on hemodialysis. The patient was an undocumented immigrant receiving health care through the emergency department as needed. Physical exam revealed pallor, pedal edema, palpable spleen, and focal tenderness over the lumbar spine. X-ray showed indeterminant compression fracture at L2, wedging of the superior aspect of L1, and deformities of the lower thoracic (T)-spines. CT scan showed (Figure 1) diffuse bony demineralization, two soft tissue deposits- one in front of the nasopharynx and other behind the odontoid process of the cervical spine (C), lytic lesions on the superior aspect of C4 and C6, compression deformity on T8 to T11, multiple lytic lesions over the lower T and the Lumber (L) spines with gross destruction of L2 spine. An urgent myelogram ruled out spinal cord compression. The patient was admitted for L2 vertebra fixation with rod and screw with instrumentation at T12, L1, L3, L4. Excisional biopsy (Figure 2) from the L2 body showed reactive bone with intervening prominent peri-trabecular fibrosis, a noticeable number of thin-walled blood vessels permeating in the bone marrow space between trabeculae, and scattered hemosiderin-laden macrophages. His admission and subsequent complete blood counts show severe anemia with Hemoglobin (Hgb) 7.2-9.5 g/dL and Hematocrit 20.9-29.4%, leukopenia (WBC 1.8-2.8 K/uL), and thrombocytopenia (Platelet 74-138 K/μL). This was managed by blood transfusions, filgrastim, and weekly darbepoetin injections without significant improvement. Peripheral blood film showed anisocytosis, poikilocytosis; the presence of macrocytes with polychromasia, elliptocytes, and dacrocytes.

Figure 1: CT contrast of the dorsal and lumbar spine: showing multiple lytic lesions at various levels, gross destruction of the L2 body.
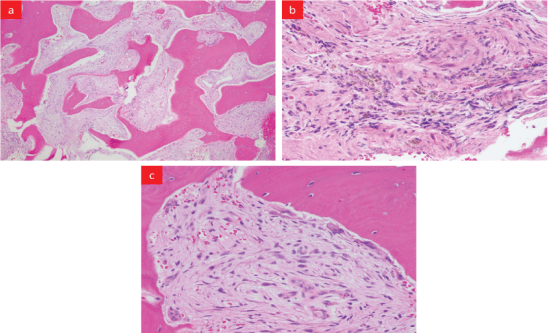
Figure 2: Biopsy of the L2 body: (a) Low power view- reactive lamellar bone with intervening prominent peri-trabecular fibrosis, noticeable number of thin-walled blood vessels permeating in between trabeculae, (b) High power view-patchy hemosiderin-laden macrophages, and (c) Lamellar bone lined by increased numbers of multinucleated osteoclasts indicative of bone resorption.
The patient’s other labs were elevated ESR (39-117 mmHg), hypoalbuminemia (3.3 g/dl) with polyclonal gammopathy (on SPEP), and elevated alkaline phosphatase 540 IU/L. His serum calcium (corrected 9-10.4 mg/dl), TSH (Thyroid-Stimulating Hormone), PSA (Prostate-Specific Antigen), CEA (Carcinoembryonic Antigen) were in normal ranges. Infectious disease workups for TB, HIV, HBV, HCV were negative. CT scan of chest, abdomen, and pelvis found diffuse bony chest wall demineralization, severe calcification of the thoracic aorta and coronary arteries globally enlarged heart, enlarged pulmonary trunk >42.5 mm, moderate splenomegaly, severe atherosclerotic calcification of abdominal aorta with its’ branches, mildly enlarged prostate gland, bladder wall hypertrophy and cortical erosion with a soft tissue deposit over symphysis pubis measuring about 3.7 cm × 4.3 cm.
Bone marrow biopsy was done to determine the cause of pancytopenia (Figure S1). The report revealed normocellular marrow with polyclonal plasma cells and extensive peri-trabecular fibrosis. Biopsy from the mass on symphysis pubis was reported to be focal granulation tissue with some hemosiderin-laden macrophages (Figure S2). Biopsy of the bladder wall revealed normal urothelium with prominent submucosal fibrosis (Figure S3).
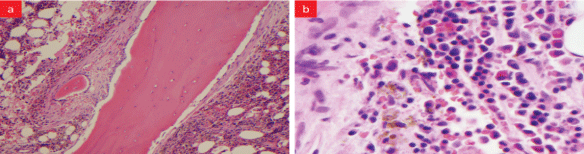
Figure S1: BM biopsy: (a) Low power view-extensive peri-trabecular fibrosis and bony remodeling with osteoblasts mostly, (b) High power view- high normocellular marrow slightly increased number of megakaryocytes, polyclonal plasma cells, and numerous hemosiderin laden macrophages.
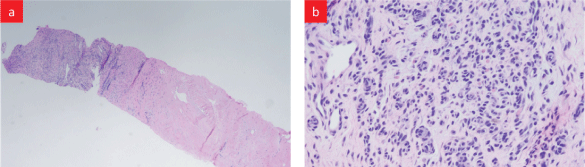
Figure S2: Biopsy from mass over pubis: (a) Low power view-benign fibrocartilage (right) with focal granulation tissue, (b) High power viewgranulation tissue with few hemosiderin-laden (brown pigment) macrophages.
Figure S3: Biopsy of bladder wall: normal urothelium and prominent submucosal fibrosis.
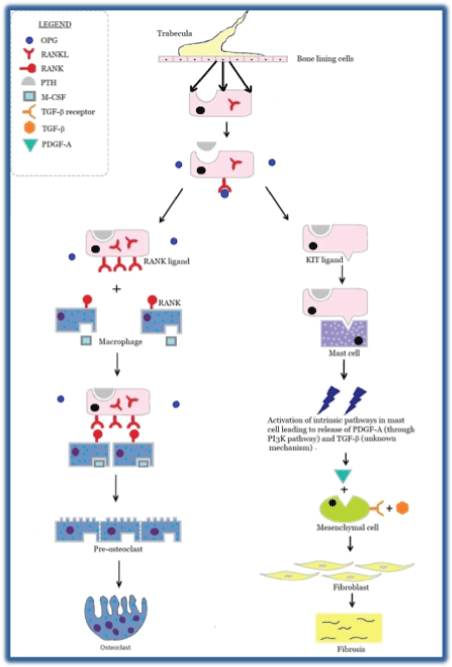
Figure S4: Represents a general overview of parathyroid bone disease in CKD. On the left side, it shows RANK-RANKL interaction with generation of osteoclast which is responsible for lytic bone lesion in HPT. On the right side, it shows KIT-KIT ligand interaction with mast cell activation and downstream fibrosis in PTH induced peri-trabecular fibrosis.
During the work-up, serum PTH-intact level was found to be significantly elevated (2020 pg/ml). An ultrasound of the neck was done showing parathyroid hyperplasia. Subsequently, the patient had a near parathyroidectomy and was discharged on clinical improvement. At 3 months follow up, the patient reported marked symptomatic improvement with Hgb stabilized to normal without blood transfusions. The patient, unfortunately, declined further follow-ups in the clinic and the future course remains undetermined.
The differential diagnosis for lytic bone lesions in mature adults >50 years old: include and are not limited to
i) Multiple myeloma, ii) Metastasis, iii) Primary bone tumor, iv) Osteomyelitis/abscess, v) Giant cell tumor (GCT) of bone, vi) Aneurysmal bone cyst, vii) Hemangioma, viii) Renal osteodystrophy etc.
High bone turnover in renal osteodystrophy can manifest as osteitis fibrosa cystica (lytic lesion), brown tumor (both lytic and expansile mass), and/or peri-trabecular bone marrow fibrosis.
There are three distinctive abnormalities associated with bone mineral disease in CKD:
1) The biochemical disorder, 2) Abnormal bone morphology and 3) Extra-skeletal calcification.
The biochemical disorders include abnormalities in i) Calcium and phosphate homeostasis, ii) Bone-specific alkaline phosphatase, fibroblast growth factor-23 (FGF23) level and serum parathyroid hormone, iii) Low active vitamin D level.
The spectrum of skeletal abnormalities seen in the CKD population includes: i) Adynamic bone disease which is characterized by low bone turnover in the setting of low PTH level; ii) Osteitis fibrosa cystica (OFC), characterized by high bone turnover, peri-trabecular fibrosis in the setting of high serum PTH (level >350-500 pg/ml); iii) Mineralization defects-for example, osteomalacia, osteopenia and osteoporosis; and iv) Mixed renal osteodystrophy characterized by a combination of high bone turnover and mineralization defect [1,2].
It is well established that hyperparathyroidism is associated with cortical bone loss. Radiologically observable bony findings of hyperparathyroidism include- i) Subperiosteal erosion of the phalanges of the hand, ii) Generalized demineralization of the skeleton resulting in lucent bone, iii) OFC which is a localized loss of all bone structures appears as the lytic lesion, iv) Brown tumors which appear as single/multiple lytic and/or blastic lesion/ lesions with or without bone expansion or deformity, v) Granular appearance of the skull due to loss of trabecular and cortical bones- “salt and pepper sign” [3].
It is shown in the animal models that in chronic hyperparathyroidism (HPTH), pre-osteoblastic cell exhibits fibroblast-like activity (due to lack of terminal differentiation into osteoblast) which tends to proliferate and migrate onto the bone surface [4]. As there is ongoing uncontrolled bone remodeling in chronic HPTH, it results in a poorly organized osteoid deposition by pre-osteoblastic fibroblast at the site of bone remodeling. This excessive deposition of poorly organized osteoid in an area of osteolysis (by osteoclast) gives rise to osteitis fibrosa cystica also known as brown tumor. Brown tumor though not a true neoplasm, is locally destructive. Histologically, a brown tumor shows fibrovascular proliferation, hemorrhagic foci, hemosiderin-laden macrophages owing to microfracture and prominent multinucleated giant cells.
Also, chronic elevation in PTH increases mast cell count in the bone marrow along with the redistribution of mast cells from bone marrow to the bone-bone marrow interface (i.e., endosteal and peri-trabecular area). Mast cell interacts with the osteoblastic cell lineage via kit-kit ligand pathway depicted in figure S4. This results in the downstream release of platelet-derived growth factor (PDGF) through activation of the phosphoinositide 3-Kinase (PI3K) along with a local accumulation of cytokines like Interleukin-6 (IL-6), transforming growth factor-beta (TGF-β), insulin-like growth factor (IGF), tumor necrosis factor-alpha (TNF-α), fibroblast growth factor (FGF), etc. These chemicals activate bone marrow stromal cells and contribute to peri-trabecular fibrosis [5].
Both Fibroblast Growth Factor-23 (FGF23) and serum phosphate play major roles in different cardiovascular outcomes in CKD [6]. FGF23 starts rising from the stage 2 CKD (eGFR between 60 to 75 ml/min/m2 BSA) whereas hyperphosphatemia becomes evident in advanced CKD particularly in stage 4 CKD (eGFR <30 ml/min/m2 BSA). Phosphate excretion in the kidney is maintained primarily by PTH and FGF23. In normal conditions, FGF23 increases phosphate excretion by decreasing the phosphate reabsorption in the proximal tubule through Klotho dependent mechanism. Due to the failure of the Klotho-dependent phosphate excretion in CKD, PTH becomes the main regulator in maintaining phosphate homeostasis. This adaptive response fails in advanced kidney disease i.e., CKD stage 4-5, and hyperphosphatemia develops despite high PTH and FGF23 levels [7]. Hyperphosphatemia causes endothelial dysfunction; osteoblastic transformation of the vascular smooth muscle cells and direct deposition of the high calcium-phosphorus product all contributes to extra-skeletal calcification (for example- calcification of blood vessels) [8].
Our patient was an undocumented immigrant, did not have an outpatient dialysis center, and thus was receiving hemodialysis on an ad-hoc basis by visiting the emergency department of whichever city he traveled through. We opine that his previous diagnosis of idiopathic cardiomyopathy with LVH s/p ICD could have been mediated by high FGF23 from long-standing untreated secondary hyperparathyroidism. It is well known that FGF23 induces cardiac myocyte hypertrophy (LVH) and is a strong predictor of congestive cardiac failure in the CKD population [6].
In our patient, the cause of anemia likely was multifactorial- i) Combination of iron deficiency, anemia of chronic disease, and ineffective erythropoiesis. Pancytopenia in this setting is also likely from ineffective hematopoiesis from defects in the release of blood cells from fibrotic bone marrow (as evidenced by high normocellular marrow in the background of high reticulocyte count, and presence of dacrocytes (i.e., teardrop cells). There have been a few case reports of pancytopenia due to myelofibrosis because of secondary HPTH due to ESRD [9]. Moderate splenomegaly in this patient’s context was likely due to myelofibrosis and was resultant from extramedullary hematopoiesis. Interestingly after parathyroidectomy in all cases, splenic size decreased with improvement in blood count. Of note, splenomegaly has been reported in both primary and secondary hyperparathyroidism [9] [SR1,R2]. [Tables S1 and S2].
| Labs | Result | Reference range |
|
Hemoglobin WBC Platelet Hematocrit Reticulocyte count |
7.2-9.4 g/dl 1.8 -2.8 x K/uL 74-138 K/uL 20.9 -29.4% 2.3% |
Range: 14 – 18 g/dl Range: 4-11 K/uL Range: 150-450/K/uL Range: 42% - 50% Range: 0.5% - 1.5% |
| MCV MCH MCHC Red cell distribution width |
97.3 31.6 33.6% 16.0% |
Range: 80 - 96 fL Range: 27 - 33 pg Range: 32 - 36% Range: 11.5 – 14.5% |
| ESR | 39-117 mm 1st hour | Range: 0 - 15 |
| CRP | 20.1 -29.1 mg/L | Range: 0.8 to less |
| Albumin | 3.45 gm/dl | Range: 3.5 – 5.5g/dl |
| S. creatinine | 2.8 – 6 mg/dl | Range: 0.7 – 1.3 mg/dl |
| BUN | 22-67mg/dl | Range: 8 – 20 mg/dl |
| Serum Na+ Serum Cl- Serum K+ Serum HCO3- Serum Ca++(corrected) Serum PO4-- |
137-141 92-101meq/L 4-6.1meq/L 20-27meq/L 8.44 - 9.84mg/dl 4-5.5 mg/dl |
Range: 136 - 145 mEq/L Range: 98 – 106 mEq/L Range: 3.5 - 5.0mEq/L Range: 23 – 28 mEq/L Range: 8.6 – 10.2 mg/dl Range: 3 – 4.5 mg/dl |
| Total Bilirubin ALT AST Alkaline phosphatase |
0.4 mg/dl 8 U/L 11 U/L 520U/L |
Range: 0.3 – 1.2 mg/dl Range: 0 – 35 U/L Range: 0 – 35 U/L Range: 36 – 92 U/L |
| SPEP | Polyclonal gammopathy | |
| Free kappa/lambda light chain ratio (serum) | 1.1 | Range: 0.26 – 1.65 |
| Serum C3 | 70 mg/dl | Range: 100 – 233 mg/dl |
| Serum C4 | 21 mg/dl | Range: 14 – 48 mg/dl |
| Serum Folate | 6.99- 8.54 | Range: 1.8 – 9.0 ng/ml |
| Serum B12 | 833 pg/ml | Range: 200 – 800 pg/ml |
| APTT | 35.8 – 39.4 secs | Range: 25 – 35 secs |
| PT | 16.1 -17.8 secs | Range: 11 – 13 secs |
| CEA PSA | 1.4 ug/ml 0.2 ng/ml |
Less than 2.5 ng/ml Less than 4 ng/ml |
| TSH | 1.540 mIU/L |
Range:0.5 – 4.0 mU/L |
| Serum Iron TIBC Transferrin saturation Ferritin |
15 ug/dl, 198 ugm/dl, 7.6% 1244 ug/L |
Range: 50 – 150 ug/dl Range: 250 – 310 ug/dl Range: 20 – 50% Range: 20 – 235 ng/ml |
Table S1: Initial and subsequent laboratory investigation.
| Cell category | Result | Comment |
| Lymphocyte gate B cells CD-19 T cells CD-3 CD4/CD8 Kappa/Lamda ratio CD-138 plasma cell gate NK cells CD3-/CD56+ CD45 dim gate Monocyte gate Granulocyte gate NRBC gate |
17% 3% 74% 3.3 1.8 0.2% 17% 1% 2% 74% 2% |
The gated population of lymphocyte is comprised of T-cells with normal expression of pan T-cell markers and normal CD4/CD8 ratio, normal proportions of NK, cytotoxic cells and polyclonal B cells. Plasma cell gate shows small population positive for CD19, mostly negative for CD56 with polyclonal light chain expression |
Table S2: Flow Cytometry of Blood.
Over time, chronic kidney disease is getting much more recognition as a multisystem disease rather than ever before. Finally, this is an excellent case illustrating the importance of having regular three times weekly hemodialysis in the ESRD population to prevent dreaded complications associated with secondary hyperparathyroidism.
1. Malignancy must be ruled out when a patient suffers from multiple lytic bony lesions with or without pathological fracture.
2. It is important to have a serum PTH level checked in patients with lytic lesions in the background of advanced CKD.
3. Diagnosis of OFC and brown tumor require constellations of biopsy from the lesion along with imaging studies, serum calcium, phosphate, and PTH levels.
4. Brown tumor is not a true neoplasm but is locally destructive.
The authors declare no competing interests.
Download Provisional PDF Here
Article Type: CASE REPORT
Citation: Chandra S, Devkota K, Bem S, Curtiss C, Swarnkar A, et al. (2021) Cancer or Not? A Case of High Turnover Bone Disease with Review of Literature. Int J Nephrol Kidney Fail 8(1): dx.doi.org/10.16966/2380-5498.222
Copyright: © 2021 Chandra S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access