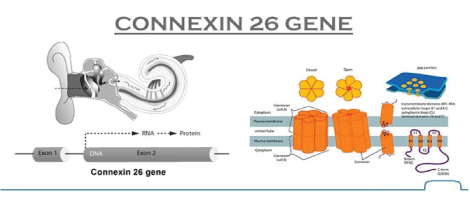
Figure 1: Molecular Structure of Connexin 26 Gene.


Muhammad Rauf1* Michael Fawzy2*
1Renal Registrar at Basildon University Hospital, UK*Corresponding author: Michael Fawzy, Basildon University Hospital, UK, E-mail: michael.fawzy@nhs.net
Muhammad Umaid Rauf, Royal Free Hospital, London, UK, Email: m.rauf@nhs.net
Connexin 26 mutation is associated with profound bilateral sensory neural deafness. Other isoforms of Connexins also form gap junctions in kidneys including endothelial cells and renal vasculature; however, Connexin 26 mutation has never been reported to be linked with acute kidney injury.
We report a case of a young patient having Connexin 26 mutation with progressive acute kidney injury on background of therapeutic dose of NonSteroidal Anti inflammatory Drugs.
Our patient is 27 years old Caucasian gentleman who was known to have background history of profound bilateral sensory neural hearing loss due to Connexin 26 mutation. He also had ongoing intermittent dizziness for past two years for which he had been referred to NeuroOtology Department at University College London (UCL).
He was brought to Accident and Emergency department of Basildon University Hospital, UK by his parents on account of severe lower back pain for two days, radiating around from umbilical region. He had returned from a skiing trip from France two days prior to the onset of pain; with no fall or trauma reported during that trip.
There was a history of flu like symptoms with runny nose around the same time, but no history of joint pains, oral or genital ulcers, rash, insect bite, fevers, dysuria, frank haematuria, diarrhoea, sore throat, any eye symptoms, chest pain or shortness of breath.
He had taken for his pain a total of 1200 mg of Ibuprofen (in 3 divided doses, 400 mg each over 48 hrs) and 90 mg of co-codamol with no benefit. He wasn’t on any regular medications or supplements and had never used steroids. He was not known to have any allergies. He was a non-smoker and never used recreational drugs while drank alcohol socially within recommended limits. He has lived with his parents, with his mother being his translator; he was independent in activities of daily living.
On presentation, he was hypertensive with Blood Pressure of 190/95 mm Hg; Heart Rate 86/min, Temperature 36.8 and Respiratory Rate of 14/min.
His physical examination revealed a young male with athletic built, in visible discomfort because of back/ abdominal pain. His abdomen, however, was soft, non-tender with no palpable organomegaly and hyper-active bowel sounds. There was no renal angle or spinal tenderness. There was no pharyngeal erythema or lymphadenopathy.
He was slightly dry with marginally reduced skin turgor. Jugular Venous Pressure (JVP) was not elevated. Heart sounds were normal with no murmur. Lungs were clear on auscultation and there was no peripheral edema. There were no stigmata of infective endocarditis.
Creatinine 294 μmol/L.
Urea 16 mmol/ L
Serum Potassium 4.2 mmol/L
Serum Sodium 138 mmol/L
Haemoglobin of 120 g/L, WBC 7.5 × 10*9/L with normal differential count including eosinophils, Platelets of 105 × 10*9/L, C-Reactive Protein(CRP) 20 mg/L , Amylase 39 U/L, normal Liver function tests , coagulation screen and normal Creatinine Kinase (CK). Adjusted serum calcium was 2.3.
He was initially being referred by Accident and Emergency of Basildon University Hospital, UK to general surgeons on account of acute abdominal/ back pain who requested a CT Urinary tract without contrast. CT scan showed no obstructive calculi or hydronephrosis in either kidney. Both kidneys were normal sized (Right 10.3 cm, Left 10.8 cm) with preserved parenchymal layer thickness. Mild splenomegaly (138 mm in long axis) was reported with no focal abnormality seen in liver, gall bladder, adrenal glands, pancreas or large and small bowel. No enlarged abdominal/pelvic lymph node and no suspicious lesion in lumbar vertebrae/pelvis noticed.
He was referred to renal team who took over the care. We have asked for repeated bloods including acute autoimmune renal screen. His creatinine increased to 516 from 294 within 24 hours. Urine dip showed 3+protein and trace blood. An abdominal X-ray was being requested at this time which identified faecal loading for which he was started on laxatives to help with constipation and morphine slow release was stopped (started on admission for back pain).
His acute autoimmune renal screen/vasculitis screen were unremarkable with negative Anti-nuclear antibodies and Anti Neutrophil Cytoplasmic Antibodies, anti-Glomerular Basement Membrane, normal complements C3/C4, normal serum immunoglobulins and decreased gamma globulins on serum electrophoresis. Creatinine Kinase (CK) was normal as well as Rheumatoid factor (RF) and Anti Streptolysin O (ASO) titre. Urine protein creatinine ratio (uPCR) was being sent. Lactate Dehydrogenise enzyme and blood film were normal with no platelet fragments; requested on account of marginally Haemoglobin and platelets (to look for Haemolytic Uraemic Syndrome, HUS).
Given the patient was hypertensive; he was started on Amlodipine 5 mg along with Intravenous hydration. His urine output was adequate.
A native renal biopsy was performed that showed acute tubular injury with no evidence of tubule-interstitial inflammation or glomerulonephritis. Mild focal scarring was reported but no significant staining for Immunoglobulin G (IgG), Immunoglobulin A (IgA) or Complement C3c (C3c) on immunohistochemistry. EM showed abnormal Basement Membrane of Proximal tubules.
His renal functions improved gradually during his admission. His abdominal pain also improved with relief of his constipation.
One week later, He was discharged with creatinine of 201 μmol/L that dropped down to his baseline of 100 µmol/L on follow up in acute kidney injury clinic few days later.
It was felt that his presentation of Acute Kidney Injury was possibly due to ibuprofen induced physiological mechanism of kidney injury on background of Connexin 26 mutations with his back pain was likely secondary to constipation, unrelated to the kidney function.
Autosomal recessive non-syndromic hearing loss is usually prelingual, non-progressive, and severe to profound.
Mutations in the Connexin 26 gene at locus DFNB1 on chromosome 13 are thought to account for about 50% of recessive non-syndromic hearing loss. 35delG is the most common mutation, but at least 90 different GJB2 mutations have been described [1] (Figure 1).

Figure 1: Molecular Structure of Connexin 26 Gene.
The gene GJB2 codes for Connexin 26, a gap junction beta2 protein. These proteins form intercellular channels in the plasma membrane and facilitate the exchange of molecules between cells. Connexin 26 is expressed in the stria vascularis, spiral ligament, spiral limbus, supporting cells of the cochlea and also in epidermis of skin. It appears to have a role in recycling of potassium [2].
Besides non-syndromic deafness, Connexin 26 mutations are also associated with Palmoplantar Keratodermas and Keratitis-icthyiosisdeafness syndrome (Figure 2).
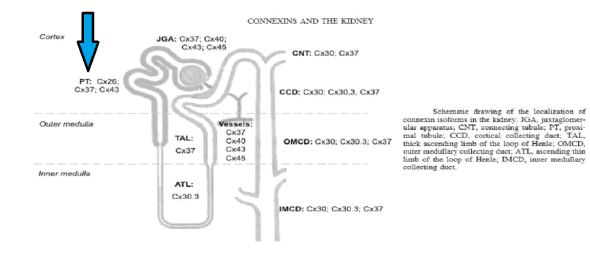
Figure 2: Distribution of Connexin isoforms in the kidney with Connexin 26 in proximal convoluted tubules.
Connexins form gap junctions in the kidney. In the renal vasculature Cx37, Cx40, Cx43, and Cx45 are expressed, with predominant expression of Cx40 in the endothelial cells and Cx45 in the vascular smooth muscle cells. However, it showed structural evidence for the presence of Cx26 gap junction plaques in the proximal tubules (PCT) which regulates 65% of sodium and water absorption (Figure 3).
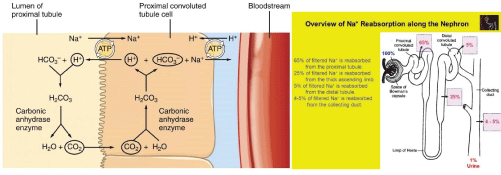
Figure 3: Proximal convoluted tubules, Sodium and water reabsorption mechanism.
Renal Connexins facilitate vascular conduction, juxtaglomerular apparatus calcium signalling, and tubular purinergic signalling. Accordingly, current evidence points to roles for these Connexins in several important regulatory mechanisms in the kidney, including the renin angiotensin system, tubulo-glomerular feedback, and salt and water reabsorption. At the systemic level, renal Connexins may help regulate blood pressure and may be involved in hypertension and diabetes [3].
Non-steroidal anti-inflammatory drugs (NSAIDs) inhibit the isoenzymes COX-1 and COX-2 of cyclooxygenase (COX). Nonselective NSAIDs inhibit both COX-1 (expressed constitutively in the kidney) and COX-2 (inducible in most tissues in response to injury or inflammation, but also present at detectable levels in normal adult mammalian kidneys), the rate limiting enzymes for the production of PGs and thromboxane (TX) [4]. COX-2 is regulated in response to intravascular volume [5]. COX-1 functions mainly in the control of renal hemodynamics and glomerular filtration rate (GFR), while COX-2 functions primarily affect salt and water excretion [6]. Blockade of either or both of these enzymes can have, therefore, different effects on renal function. In a person with normal renal hemodynamic parameters, PGs do not play a dominant physiologic role in maintaining renal blood flow and GFR [7].
Certain Non-steroidal anti-inflammatory drugs (NSAIDs), such as sulindac, ibuprofen, and flurbiprofen may exert antiinflammatory effects independently of COX activity and prostaglandin synthesis. Those anti-inflammatory effects are mediated by inhibition of certain transcription factors such as activator protein 1 and nuclear factor-κB and/or by alterations in the activity of IκB kinase, mitogen-activated protein kinase, and cyclin-dependent kinase [8].
Based upon these facts, In Cx26 mutation case, sodium and subsequently water absorption get impaired from PCT which was exaggerated by Non-steroidal anti-inflammatory drugs (NSAIDs) afferent vasoconstriction that was eventually sensed by the kidney as profound hypo-perfusion and Acute Tubular Necrosis (ATN) like picture. We believe that increased renin angiotensin which caused high BP despite of being marginally intra-vascular depleted.
The association between Connexin 26 mutation and its role in disruption of hemodynamics of kidney was proved by ATN picture in kidney biopsy which can’t be explained by sole culprit and abnormal basement membrane junctions of PCT on EM.
It’s first reported case for association between Connexin 26 mutation and Acute Kidney Injury in usual setting of young patient with no other comorbidities receiving therapeutic dose of Nonsteroidal anti-inflammatory drugs (NSAIDs). Albeit, we suggest that more molecular analysis needed to define more possible association between Connexins mutations and kidney problems.
Download Provisional PDF Here
Article Type: CASE REPORT
Citation: Rauf M, Fawzy M (2019) Coupling of Acute Kidney Injury with Connexin 26 Gene Mutation. Int J Nephrol Kidney Fail 5(2): dx.doi.
org/10.16966/2380-5498.172
Copyright: © 2019 Rauf M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access