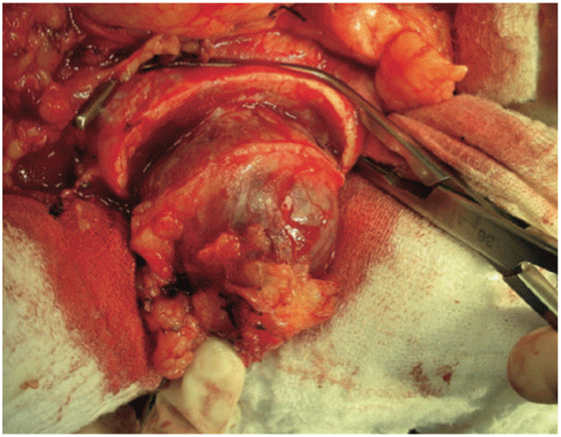
Figure 1: Vascular clamp in place after tumor resection (Original photograph from the first article published by this group of investigators) [17].


Wagner Eduardo Matheus* Marcelo Cartapatti da Silva Aline Akel Ferruccio Marcelo Antunes de Souza Fernandes Denardi Ubirajara Ferreira
Department of Urologic Oncology, Universidade Estadual de Campinas-UNICAMP, Campinas, Sao Paulo, Brazil*Corresponding author: Wagner Eduardo Matheus, Department of Urologic Oncology, Universidade Estadual de Campinas-UNICAMP, Campinas, Sao Paulo, Brazil, Tel: +55 (19) 3521-2121; E-mail: wematheus@uol.com.br
Partial nephrectomy is the first treatment option for stage T1 renal tumors based on international consensus; however laparoscopic radical nephrectomy is the chosen technique in most urology communities nowadays, due to new technologies available, but poor knowledge of laparoscopic techniques or open surgery advantages. Open partial nephrectomy is a valuable choice that needs to be reinforced, even more so when nephron-sparing techniques with selective renal parenchymal clamping have been proposed and proved applicable, maintaining oncological outcomes and reducing surgical comorbidities.
The present retrospective study reviewed a series of 64 patients with localized renal tumors who were operated using this modified partial nephrectomy to gather more data on the surgical technique proposed by Denardi, et al. in 2005. The results sustain literature’s findings, showing low peri-operative complication rates and optimal long-term oncological outcomes. Therefore, partial nephrectomy with selective parenchymal clamping is a surgical possibility to be thought according to the situation and further studies are required to properly define its advantages and disadvantages compared to the other available techniques.
Nephrectomy; Nephron-sparing; Clamping; Warm ischemia; Renal tumor
The widespread use of medical imaging in numerous health disorders has resulted in an increase in the incidental diagnosis of localized renal tumors [1,2]. Under these circumstances, it is now an international consensus in the urology community that the first treatment option for stage T1 renal tumors (i.e. those<7 cm) should be partial nephrectomy (PN), which provides oncologic outcomes comparable to those obtained with radical nephrectomy (RN) with less impact on renal function [3-7].
Meanwhile, with the advance of minimally invasive procedures such as laparoscopy and robotics, laparoscopic radical nephrectomy (LRN) has become the recommended gold-standard surgery for localized renal tumors. Laparoscopic PN is only feasible by skillful hands and indicated for selected patients with small peripheral tumors, therefore, radical approaches have more popularity. PN by open surgery presents itself as the viable option in most cases [8-10]. Recent studies have found an improvement in the recovery of renal function following surgeries in which the warm ischemia time was shorter, with 30 minutes being considered ideal [1,11-13]. To improve surgical performance and remove the tumor completely, it is usually necessary to access the hilum and clamp the artery to avoid substantial bleeding [14-16].
In 2005, Denardi, et al. published a case series of 17 patients with early-stage renal tumors who were submitted to nephronsparing surgery with selective renal parenchymal clamping. In these cases, the drawbacks of mandatory arterial clamping were minimized [17]. The goals of this technical modification to conventional PN were to minimize kidney dissection, reducing the surgical procedure time and blood loss, as well as eliminating ischemia in the remaining renal tissue, conserving the optimal oncological outcome and reducing parenchymal damage.
The present study reviewed a series of 64 patients with localized renal tumors who were operated using this modified PN surgical technique. The results reported here provide new data on long-term results of this procedure.
This study provides an update on PN with selective renal parenchymal clamping, a technique first described by Denardi, et al. [17]. A retrospective review was conducted including data from all patients that were submitted to PN between January 2005 and June 2013 at University of Campinas’ Hospital in Campinas, Brazil. The same group of urologists performed all the surgical procedures.
The data collected from the patients’ records included their demographic characteristics such as age and sex, any comorbidities (Table 1), pre- and post-operative renal function, tumor size and location, operating time, blood loss, time until hospital discharge and any complications that occurred during the procedure or follow-up. Patients to whom had been considered PN but for some reason the procedure was contraindicated were excluded from this study.
| (N) | % | |
| Men Women |
33 31 |
51.5 48.5 |
| Mean value | Range | |
| Age | 52.2 | 17-82 |
| Lifestyle and Co-morbidities | (N) | % |
| Smoker Systemic arterial hypertension Dyslipidemia Diabetes mellitus Chronic kidney disease None |
15 25 9 9 5 19 |
23.5 39 14 14 7.8 29.7 |
Table 1: Demographic, clinical and lifestyle-related data of the patients evaluated.
Preceding the surgery, a physical examination was performed, laboratory evaluation of hemoglobin and hematocrit levels, kidney and blood clotting functions were carried out, as well as cardiac and respiratory functions were assessed. Most of the tumors were diagnosed by abdominal ultrasonography; however, all patients were referred for a pre-operative CT scan or MRI to allow a better anatomical evaluation.
All the procedures were performed under general anesthesia. According to the size and site of the tumor, an anterior subcostal incision or lumbotomy was performed.
With this technique, after accessing the retroperitoneal space, the kidney is minimally dissected, with only en bloc separation of the perirenal fat and kidney from the surrounding tissues [17]. Subsequently, Gerota’s fascia is sectioned and perirenal fat should only be dissected 1 or 2 cm from the tumor edges. After completely exposing the lesion, parenchymal clamping is initiated. In our institutes, a vascular clamp (Satinsky clamp) is used, providing secure compression with minimal parenchymal damage (Figures 1 and 2). While resecting the tumor, clear surgical margins must be ensured and negative macroscopic margins are considered to be sufficient. The intra parenchymal vessels and opened collecting system are closed with absorbable suture (polyglactin 3-0). The perirenal fat flap is used to seal the remaining parenchyma, which is sutured edge-to-edge using absorbable polyglactin sutures (Figure 3). A Penrose drain is placed to allow control of the blood loss.

Figure 1: Vascular clamp in place after tumor resection (Original photograph from the first article published by this group of investigators) [17].
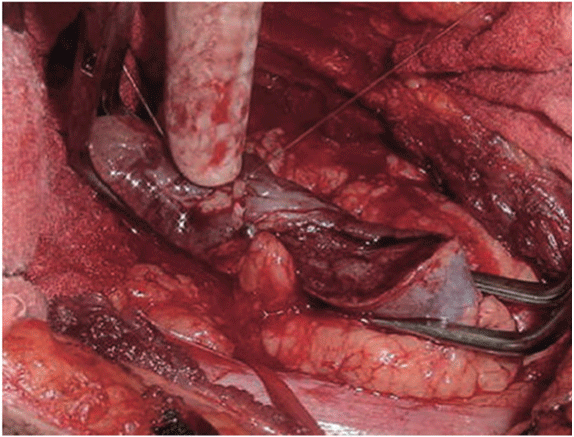
Figure 2: Vascular clamp in place after the lesion has been resected and small vessels and collecting system have been sutured.
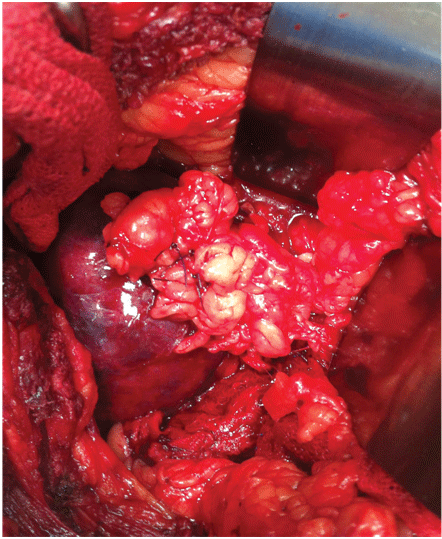
Figure 3: A flap of perirenal fat sutured within the parenchyma for hemostasis.
Seventy patients were meant to be submitted to PN during this study; however, six were excluded from the analysis because the procedure had to be changed to RN, either because the tumor was located too close to the renal hilum or the tumor size had been underestimated in previous imaging exams. Therefore, only 64 patients remained in the study: 33 men (51.6%) and 31 women (48.4%). The mean age of the patients was 52.2 years (range 17-82 years). In 24 patients (37.5%), the left kidney was involved, whereas in 39 cases (60%) the tumor affected the right kidney and in one patient both kidneys were compromised. The mean tumor size was 3.95 cm (range 1.8- 8.0 cm). The mean surgical procedure time was 156.7 minutes (range 60-315 minutes) and the mean volume of blood lost was 360 ml (range 100-1500 ml). Only six patients (9.4%) required a blood transfusion. The mean duration of hospitalization was 3.6 days (range 2-10 days).
Histology revealed most of the tumors to be malignant (76.6% of all cases), with 40 cases of clear-cell renal cell carcinoma (62.5%), 7 of papillary renal cell carcinoma (10.9%) and 2 of chromophobe renal cell carcinoma (3.1%). With regard to benign lesions, there were six cases of renal oncocytoma (9.4%), five cases of benign renal cysts (7.8%) and four cases of renal angiomyolipoma (6.3%). The characteristics of the tumors are shown in Table 2. Surgical margins were positive in only one case in which a 56-year-old man had a 4.5 cm clear-cell renal cell carcinoma, Fuhrman grade 1-2, in his right kidney. After 36 months of follow-up, CT scans showed no tumor recurrence or metastasis.
| Diameter (cm) | Range | |
| Size | 3.95 | 1.8-8.0 |
| Location | (N) | % |
| Upper pole Lower pole Middle Left Right Bilateral |
28 19 17 24 39 1 |
43.7 29.7 26.6 37.5 60.9 1.6 |
| Histology | (N) | % |
| Clear-cell renal cell carcinoma Papillary renal cell carcinoma Oncocytoma Benign cyst Angiomyolipoma Chromophoberenal cellcarcinoma |
40 7 6 5 4 2 |
62.5 10.9 9.4 7.8 6.2 3.2 |
Table 2: Tumor characteristics.
Complications were rare and were classified as acute (occurring before the patient was discharged from hospital) or long-term. The most common acute complication was bleeding requiring a blood transfusion; however, this accounted for less than 10% of all cases. Acute renal failure occurred in only two cases (3.1%), neither of which progressed to chronicity. In another two cases, a repeat intervention was required, in one case due to uncontrollable bleeding leading to RN and the other due to evisceration. Long-term complications were rare, with two patients developing incisional hernias and only one progressing to end-stage renal failure requiring dialysis. In this latter case, the patient had stage 4 chronic kidney disease prior to surgery.
Over a mean follow-up period of 29.9 months (range 6-96 months), only two patients developed metastasis. In both cases, the tumor size was >6 cm, Fuhrman grade 2-3, and the surgical margins were negative.
The role of PN has grown in importance in the past few decades due to the increasing number of tumors diagnosed at early stages [1,2]. This surgical technique has become valuable nowadays when urologists are facing the need to achieve better oncological outcome with less kidney damage as an era of improved diagnostic imaging technology and less restricted indications for nephron-sparing procedures has begun. However, in non-referral medical services throughout Brazil, laparoscopic RN generally is the indicated procedure, even in cases where PN would be a much more suitable technique. This fact counter poses the technological and human resources scenario of the country, where the use of laparoscopy for urological procedures is still not widespread to all hospitals. In addition, most urological services that include laparoscopy do not have all the material necessary to perform a procedure such as laparoscopic PN, which is technically more challenging. Thus, it is clear that the great obstacle for performing the PN technique approached by this study is that not all urologists are familiar with nephron-sparing procedures and prefer radical surgery. The poor knowledge of this technique reflects on the patients’ surgical outcome, since radical approaches are linked to more complications, while several studies comparing PN with RN have shown similar oncological efficiency. In a retrospective case series of 3,480 patients with clinical stage cT1a renal tumors, Antonelli, et al. reported 5 and 10-year cancer-specific survival rates of 94.7% and 90.4%, and 96.1% and 94.9% for PN and RN, respectively [18]. In 2010, a large population-based study conducted using the SEER database and published by Crepél, et al. reported similar oncological cure rates with PN and RN for pT1bN0M0 renal cell carcinomas [19].
In a world where modern techniques have greater appeal, some may advocate that laparoscopic and robot-assisted PN will soon supplant open surgery as the gold standard procedure for small renal masses [1,4,14]. We strongly support the hypothesis that open PN will always play its role, since only a small percentage of hospitals of an immense country such as Brazil have the resources required to accomplish that goal. Moreover, it is difficult for physicians who are not working in a referral care institute to acquire the skills needed to perform these new procedures. An international, multicenter, retrospective, paired study conducted in 19 hospitals in Europe compared open PN with robot-assisted PN for renal cell carcinomas with respect to peri-operative, early oncological and functional outcomes [20]. That study found no significant differences between the two techniques, thus corroborating our theory on the continued use of open PN.
A multicenter retrospective study conducted in Italy (RECORd Project) evaluated a series of 450 patients undergoing open or laparoscopic PN. No significant differences were found of surgical procedure time (131 vs 143 minutes) or blood loss (221 vs 164 ml) (p>0.05), respectively. However, in a multivariate analysis, laparoscopic PN had a longer warm ischemia time (WIT) [19.9 vs 15.1 minutes, p<0.001] and a risk 6.3 times higher of WIT >25 minutes compared to open surgery [1]. It is known that the longer the WIT, greater the damage to renal parenchyma [1,11-13]. Therefore, the present technique represents an advance towards the goal of zero ischemia, since selective parenchymal clamping requires only enough compression to contain minor parenchymal bleeding (pressure that can easily be controlled manually by the surgeon), with the renal hilum remaining intact. Finally, the improvements observed in the PN made open as the study described could be implemented in the laparoscopy approach by applying the parenchymal clamping technique to the usual PN procedure, but further studies are needed to reach conclusions on its efficiency.
Recently, Villavicencio et al. reported on the use of an offclamp technique in 19 cases of retroperitoneoscopic partial nephrectomy for localized renal tumors. Those authors reported a mean blood loss of 414 ml (range 100-1600 ml), mean WIT of 4.9 minutes (range 0-28), mean surgical procedure time of 182 minutes (range 110-285 minutes) and mean hospitalization of 4.5 days (range 3-11 days) [14]. These results are in agreement with our own statistics, making it reasonable to speculate that our technique has consistently proven to be comparable to the minimally invasive procedures, which are only feasible by experienced hands in a referral institute. Positive surgical margins are present in up to 7% of cases of open PN, with fewer results in laparoscopic or robot-assisted surgery [21]. In the present study, only one case (1.6%) was managed conservatively due to prior chronic renal disease, showing no signs of metastasis or local recurrence during follow-up.
The institutions in which this work was performed offer a residency program and this may explain the large variation in peri-operative results. In the case of this particular technique, the learning curve is relatively small and a good outcome can be achieved after a relatively limited number of procedures. Despite the retrospective nature of this study, it is possible to affirm that this technique is safe and provides optimal oncological control with minimal physical damage to patients. Prospective randomized studies should be conducted to compare this technique with others in order to ratify the role of nephronsparing surgery with selective renal parenchymal clamping as an effective and safe treatment option for renal masses.
In conclusion, the present technique represents a safe treatment option for localized renal tumors, with low perioperative complication rates and optimal long-term oncological outcomes. However, further studies are required to confirm these findings.
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Matheus WE, da Silva MC, Ferruccio AA, de Souza MA, Denardi F (2018) Nephron-Sparing Surgery for Renal Tumors Using Selective Renal Parenchymal Clamping: Long-Term Results. Int J Nephrol Kidney Fail 4(2): dx.doi.org/10.16966/2380-5498.159
Copyright: © 2018 Matheus WE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access