Figure 1: Flow diagram


Yvette AH Matser1 Matty L Terpstra2 Frederike J Bemelman2*
1Department of Nephrology, VUmc School of Medical Sciences, Amsterdam, Netherlands*Corresponding author: Frederike J Bemelman, Department of Nephrology, Academic Medical Centre, Amsterdam, Netherlands, Tel: 0031-20-5665990; Fax: 0031-20-5669241; E-mail: f.j.bemelman@amc.uva.nl
Organ transplantation from donor to recipient carries an inherent risk of disease transmission. Organ donors with a history of breast cancer are classified as non-eligible because of their risk of transmitting cancer to the recipient, even many years after curative treatment. However, recipients waiting for organ transplantation endure significant risk of death. The number of living donations is increasing, and the donor pool would expand if we include donors with a history of breast cancer, as it is one of the most common forms of cancer diagnosed in woman. Whether organs from donors with a history of breast cancer should be accepted remains a topic of debate. In this systematic review, we provide a structured overview on the current available literature on the transmission risk of breast cancer after transplantation. This can help to determine whether it is safe to accept donors with a history of cancer. The search resulted in a total of 12 studies with 142 donors with a history of breast cancer. Our study shows that transmission did not occur when donors had a history of breast cancer, while it did occur when donors had present breast cancer. If we compare these results with the mortality rate while awaiting transplantation, the risk of dying while waiting for an organ is much higher than the risk of breast cancer transmission. Careful risk-benefit assessment and close collaboration between transplant teams and specialists can increase the the chance of survival with a low risk of cancer transmission.
Cancer Transmission; Donor Derived Cancer; Organ Donation; Kidney Transplantation; History of Cancer
CTTR-Cincinnati Transplant Tumor Registry; DCIS-Ductal Carcinoma In Situ; DTAC-Disease Transmission Advisory Committee; IPITTR-Israel Penn International Transplant Tumor Registry; LCIS-Lobular Carcinoma In Situ; OPTN-Organ Procurement and Transplantation Network; PHC-Past History of Cancer; UNOS-United Network for Organ Sharing.
There is still a shortage of organ donors, with 22 patients dying every dayin the United States while waiting for a transplant [1]. The gap between the supply and demand for donor organs might merit extending donor criteria. According to current guidelines, malignancy in a potential donoris a contraindication for organ donation, except for most skin carcinomas and some localized tumours, such as intracerebral malignancies [2,3]. Accepting organs from donors with a history of cancer on the other hand, is an increasing topic of debate.
One possibility to reduce the organ shortage may be to include donors with a history of cancer [4,5,6]. However, this carries the risk of transmitting cancer to the transplant recipient [7,8]. The OPTN/UNOS reviewed 39 455 cases of deceased donors from 2000 to 2005, of whom868 (2.2%) had a history of cancer. Yet, transmission of cancer only resulted in four deaths among recipients. In this time period there were 39 519 patients who died waiting for an organ donor [9].
If the risk of cancer transmission is carefully outweighed, these organs could be included in the donor pool or could perhaps be used in urgent situations [9]. This would both reduce the length of the donor waiting list and decrease the number of patients that dies while waiting for an organ.
In this review we focus only on kidney donors, because the number of living kidney donations is rapidly increasing [10]. A substantial amount of organ donors can be gained if patients with a history of breast cancer would be included in the donor register, as it is the most common form of cancer diagnosed in women in the United States [11]. However, breast cancer is notorious for its late recurrence [12]. According to the literature, only donors with non-invasive forms, such as ductal carcinoma in-situ (DCIS) and lobular carcinoma in-situ (LCIS), should be used as donor [13]. If we look at invasive forms of breast cancer, the recommendations for donors with stage T1a or T1b, without lymph node involvement is a disease free interval before donation of more than 10 years [14].
This study systematically reviews all published data on the transmission of breast cancer from donors to renal transplant recipients in order to answer the main question: what is the transmission risk when transplants from donors with a history of breast cancer are being used?
We conducted a systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [15]. The methods for this systematic review were specified in advance and documented in a review protocol. This protocol was registered with PROSPERO and can be accessed through registration number, CRD4017058773.
Studies were included if they reported data on breast cancer transmission in recipients who received a donor kidney from deceased and/or living donors, who had a history of breast cancer or a present cancer at the time of donation. Malignancies in the recipients were only considered as donor derived if this was histopathology-proven. All study types that reported data on this topic were included, except for reviews.
The articles were identified using electronic databases Medline and EMBASE with the following key search terms: “kidney transplantation/,” “tissue donors”, “cancer” and “malignan* ”. Appendix S1 shows the search strategies that are used. An additional search using Google Scholar for finding the grey literature on this subject was performed. We restricted our review to papers published after 2012. We used the systematic review from Xiao, 2012 and other articles with the corresponding references to find relevant studies published before 2012.
Titles and abstracts were independently screened by two reviewers (YM and MLT). Possible relevant articles were reviewed by reading the full text. Articles evaluating the occurrence of non-donor derived malignancies after transplantation were excluded. Relevant information was extracted out of the data collection such as: the cohort, the number of kidney donors with a breast cancer history, donor characteristics (Living/ Deceased, Related/Unrelated), disease free interval of the donor, cancer transmission rate, type of the cancer in the recipient, the time before the manifestation of the cancer in the recipient and if the cancer is proven to be of donor’s origin.
The primary out come for this review is the breast cancer transmission risk from donors, living or deceased, with a history of breast cancer. The secondary outcomes are; the mortality rate in the recipients, the nature and treatment of malignancy in the donor and the interval between diagnosis and organ donation and between organ donation and cancer development in the recipient. Due to the heterogeneity in the data of the included studies, it is not possible to perform a meta-analysis.
The quality of the included studies was critically appraised through the Newcastle-Ottawa quality assessment [16] for cohort studies, for assessing the selection of participants, the comparability of cohorts and assessments of the outcome. The Newcastle-Ottawa scale was ranked with stars, shown in appendix S2. Since our search strategy was partly building on the systematic review from Xiao [17], we also performed a quality assessment of this article through the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist [15]. Each item in the PRISMA form was ranked was yes, incomplete or no, shown in appendix S3.
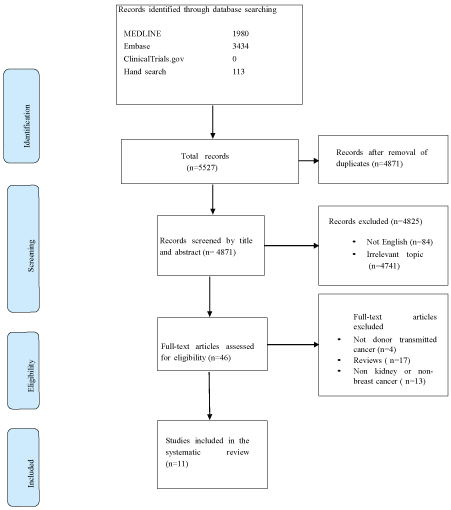
The search resulted in a total number of 5527 articles, 656 articles appeared to be duplicates and 4825 articles were ineligible after screening the title and abstract. The remaining 46 articles were reviewed in full text form; with eventually 12 cohort studies included in this systematic review (Figure 1). We contacted 2 authors of our included studies for additional information, but we did not receive a response.
3
Figure 1: Flow diagram
The quality assessment of our included studies is also shown in Table 1. The selection of a non-exposed cohort was not applicable in our included studies. All studies controlled confounders by separating in subgroups between different types of organs and/or different types of cancers in the donor.
| Study | Cohort and donors (n) | Kidney donors with BCH (n) | Donor characteristics | Breast cancer Stage in the donor | Donor’s disease free interval | Recipient tumour | Time until manifestation cancer in the recipient |
Transmission rate |
PA proven |
Quality appraisa l |
| Kauffman et al. (2000) [2] | OPTN/UNO S 257 PHC (total cohort: 14.705) |
26 | Cadaver | NR |
76.3% > 5 years |
PTLD (n2) BCC (n1) | Not until 61 months after donation | 0/26 | Not donor derived | 8* |
| Kauffman et al. (2007) [9] | OPTN/UNO S 1069 PHC (total cohort: 39.455) |
69 | Cadaver | NR | > 34.2% < 10 years > 55.6% > 10 years |
None | - | 0/69 | - | 5* |
|
Penn et al. (1991, 1988, 1995) [4] Buell et al. ( 2 0 0 4 ) [13] |
CTTR 179 donors with PHC or present cancer (Total cohort: NR) |
7 |
Living (3n) & Cadaver (4n) |
Invasive breast cancer – Stage NR | Treated < 10 years before donation, or were found to have neoplasia at the time of donation, or developed evidence of it within 18 months after donation. |
N1: tumour in allograft. Death unrelated to the cancer N2: metastases found in specimen. |
N1: tumour at autopsy 2 weeks after transplantatio n N2: Ntx after 5 days due to infection. The recipient remained tumour free |
2/7 | Histological ly identical to those in the original donors | 5* |
Table 1: characteristics of the included studies (n=4)
PHC = past history of cancer, BHC =breast cancer history, PTLD = post-transplant lymph proliferative disease, BCC = basal cell carcinoma, PDDTE = potential donor derived transmission events, NR= Not reported
A total of 12 registry studies were included and compared with each other, as shown in in table 1. Only the kidney transplant recipients with a breast cancer history were included. The included studies were carried out in different countries: United States (n=7), Denmark (n=1), United Kingdom (n=2) and Germany (n=1). If more articles used the same cohorts of the OPTN/UNOS or the IPITTR, the most recent article was included to extract the data.
A total of 142 donors (deceased=126, living=6, not reported=10) with a history of cancer or occult present cancer donated a kidney. The stage and type of the donor’s cancer is important for determining the risk of transmitting the breast cancer. Unfortunately, the majority of articles did not report this information. In most of the donors the cancer free interval before donation was more than 5 years.
The studies prove that the transmission of breast cancer does not happen if only the donors with a history of breast cancer are included. However, these studies reported a long disease-free interval before donation.
Transmission only occurred in studies in which donors with a present malignancy had been included. The study from Penn reported a transmission rate of 29%, but this was related to invasive breast cancer, of which the stage of invasive breast cancer is not reported. Although 142 donors were included, tumour transmission was only found in three recipients. All tumor transmissions were histologically proven to be of donor’s origin.
This systematic review shows that the transmission of breast cancer does not occur if the donor had a history of breast cancer with an extended disease-free interval. In the described cases where transmission of breast cancer did occur, the time between the occurrence of the malignancy in the donor and the organ transplantation was less than 10 years. In some cases the malignancy was even present at the time of donation. The inclusion criteria of Penn’s study were donors with: a history of breast cancer within 10 years before donation, an apparent neoplasm at the time of donation or manifestation of breast cancer in the donor within 18 months after donation (thus only living donors). It remains unclear if the donors who transmitted breast cancer had a malignancy at the time of donation. This supports our conclusion that a disease-free interval of more than 10 years and non-invasive forms of breast cancer could be considered for transplantation, whilst the presence of breast cancer at the time of donation is an absolute contra indication for donation because of the high transmission risk. None of the included studies reported data on the transmission of more advanced stages of breast cancer with a shorter disease-free interval.
Several other reports also described recommendations for donors with a history of breast cancer. Adams (University of Virginia) reported the risk for tumour transmission categorized by the stage of breast cancer in the article from Feng et al. (2003). The risk for tumor transmission categorized by the stage of breast cancer. The arguments are based on the existing literature about the long term follow up of survival in breast cancer patients with stage I and stage II [18]. They conclude that for stage 0 breast cancer no disease-free interval is necessary. Further, for stage T1a or T1b they recommend a disease free interval of 10 years or more and for breast cancer T1c they advise to not accept donations [6]. Our review supports the idea to always adhere to the 10 years disease free interval for safe transplantation.
Furthermore, the article by Nalesnik, et al. (2011) and Zhang, et al. (2014) also presents guidelines for the difficult decision of including these potential donors by categorizing risk categories. Breast cancer stage 0 in situ is defined in the intermediate risk category (1-10% transmission risk) and present breast cancer>stage 0 is associated with a high risk (>10% transmission) according to the DTAC Malignancy Subcommittee. They conclude that exceptions may be made for patients with a history of breast cancer with non-invasive forms such as ductal carcinoma in-situ (DCIS) and lobular carcinoma in-situ (LCIS) who have had an extended diseasefree interval [19,20].
In summary, the above mentioned studies all conclude that there is a low incidence of tumor transmission in (I) early stage breast cancer and (II) in case of a long disease free interval. This justifies the utilization of organs from these donors. The presence of breast cancer at the time of donation forms a high risk of transmission. These finding will help to create a framework to assist in the decision-making process.
If transmission occurs, graft nephrectomy and cessation of immunosuppression are the two most common forms of therapeutic and surgical interventions. Kauffman (2002) reported the presence of breast cancer at the time of donation that resulted in transmission from wife to husband. Six months post-transplant metastases were found in the recipient due to ductal breast carcinoma. Immunosuppression was stopped, the graft remained in situ and chemotherapy was started. The patient rejected the tumour and the graft. Against expectations, the donor remained tumour free during the 4 year follow-up and has been listed again for an cadaveric kidney transplantation [4,17].
This systematic review has several strengths. To our knowledge, this is the first systematic review that focusses entirely on the transmission of breast cancer from donors with a history of different breast cancer stages and varying disease-free intervals. We used a systematic approach with quality assessments for the included articles to provide valuable evidence regarding the outcome of using donors with a breast cancer history.
However, our study has some limitations. Firstly, the literature on cancer transmission is very limited. The only available literature consists out of registry studies, research reports and retrospective studies. Clinical trials are beyond the bounds of possibilities because of the ethical issues involved in using extended donor criteria.
Furthermore, the available data in the registry studies was uncomprehensive and undetailed, which makes it harder to draw conclusions. The lack of consistent reporting made comparison between the included studies unfeasible. The studies reported variations in follow up time in the recipient and donor’s cancer free interval. The cancer stages and related treatments were often not reported; however, they are important for calculating risk evaluations.
There is an increasing need to balance the risk of using organs from donors using extended donor criteria, including those who may potentially transmit their disease to recipients, to expand the donor pool. The continual shortage of donor organs, just as the number of patients waiting and dying on the kidney transplant waiting list. The Euro transplant Statistics Report in 2016 describes 6586 kidney waiting list registrations and 604 mortalities while waiting for a kidney, resulting in an annual mortality of 9.17% in the countries covered by Euro transplant [21]. The risk of dying while waiting for a kidney is much higher than the risk of developing a donor derived breast cancer, so a cautious approach may no longer be appropriate in all cases looking at the statistics.
This review can aid transplant teams and recipients in the decisionmaking process when considering using organs from donors with a history of breast cancer after risk benefit assessments. Perhaps potential recipients should be involved in this process and be fully informed about the risks of cancer transmission through donation.
The decision to include donors is sometimes difficult, because the agreement on including potential donors has to be made quickly, based on donor information that may be incomplete or requires additional information.
National and international transplant registries with complete and accurate information on donor and recipient characteristics should be used for future studies to calculate the exact transmission risk and make a true risk-benefit assessment for each individual patient.
Download Provisional PDF Here
Article Type: Research Article
Citation: Matser YAH, Terpstra ML, Bemelman FJ (2017) Cancer transmission risk from kidney donors with a history of breast cancer: a systematic review Int J Nephrol Kidney Fail 4(1): doi http://dx.doi. org/10.16966/2380-5498.151
Copyright: © 2017 Matser YAH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access