Figure 1: Study Protocol


Krishan L Gupta1* NavinPattanashett1 Jai Babu2 Usha Dutta3
1Department of Nephrology, Postgraduate Institute of Medical Education and Research, Chandigarh, India*Corresponding author: Krishan L Gupta, MD, DM. Diplomate NB. (Neph), MNAMS, FISN, FICP, Professor of Nephrology, Postgraduate Institute of Medical Education And Research, Chandigarh -160 012 (India) Tel: no.91-172-2756732 (O) - 2700070 (R); Fax: 91-172-2749911/ 2740044; E-mail: klgupta@hotmail.com
Introduction: Gastrointestinal manifestation of systemic lupus erythematosus varies widely. Abdominal pain vomiting and diarrhoea are seen in more than 50% SLE patients. Many of them are nonspecific and occur either as direct involvement of the GI tract or the effects of various medications.
Methods: This is two year prospective study; patients diagnosed as SLE based on American College of Rheumatology criteria were included. Detailed assessment of history, clinical examination and investigations were done. Gastrointestinal and hepatic symptomatology was scored based on clinical profile. And the score was correlated with findings on upper gastrointestinal endoscopy (UGIE) with biopsy, manometry, sigmoidoscopy, ultrasound abdomen and liver function tests.
Results: The study included 32 patients, 2(6.2%) males and 28(93.8%) females. The mean age was 26.2+7.7 years (17 - 64 years). 30(93.75%) had gastrointestinal symptoms and 15/28 patients (53.3%) showed abnormal findings on UGIE. The pattern of abnormality were, hyperaemia in lower end of esophagus in 6, mild gastritis in 11, atrophic gastritis in 4, antral gastritis in 9 patients. On esophageal manometry, 4/15 (26.67%) showed esophageal dysmotility. GI mucosal histopathology was abnormal in 19 patients (66.6%). 11(39.3%) had atrophic gastritis and 8 (28.6%) had chronic inflammatory infiltrates (3 H.pylori infection and 5, no specific etiology).When GI symptom scoring compared with UGI endoscopy, score ≥ 8 showed significant correlation with abnormal UGI endoscopy and histopathological findings (ρ =0.047).
Conclusion: SLE may present with protean GI manifestations. Majority of them are mild. Sever forms of GI symptoms warrants invasive investigation like UGIE and biopsy, and they significantly correlated with histopathological findings.
Gastrointestinal manifestations in SLE were first described by Sir William Osler in 1895 [1]. Abdominal pain, vomiting, and diarrhea are seen in more than50% SLE patients [2]. Many of these symptoms are nonspecific, and often reflect either direct involvement of the GI tract by SLE or the effects of various medications.
Dysphagia is the most frequent complaint and is usually due to esophageal hypomotility and it may also be caused by esophageal stricture and gastroesophageal reflux disease (GERD), esophageal candidiasis, vasculitic ulcers and pill esophagitis. Abdominal pain accompanied by nausea and vomiting occurs in up to 30% patients with SLE. Dyspepsia has seen in 10-50% of patients, while peptic ulcer has seen in 4-21%. These complications are more common in patients treated with non-steroidal anti-inflammatory drugs and Glucocorticoids.
Acute abdomen with nausea, vomiting, diarrhea, GI bleed, and fever, secondary to mesenteric vasculitis may occur. Pancreatitis occurs in around 2 to 8 percent of patients, it may result from vasculitis or thrombosis (often in association with antiphospholipid antibodies). Protein-losing enteropathy typically occurs in young women, may represent the first manifestation of SLE. Diarrhoea is present in 50% of patients.
Hepatic dysfunction occurs in many patients with SLE. However, most of them are mild, non-specific and asymptomatic. They can be caused by SLE itself or drugs used for treatment.
It’s a two-year prospective study, SLE patients who satisfied the American College of Rheumatology (ACR) criteria [1] were included. A detailed assessment of history, clinical examination and investigations were done. The study protocol and design mentioned in Figure 1 and 2 respectively. Gastro-intestinal and hepatic symptomology were scored based on their clinical profile (supplementary table), and the score was correlated with findings on Manometry, Upper gastrointestinal endoscopy with biopsy, Sigmoidoscopy, Ultrasound abdomen and Liver function tests. The study was approved by institute ethic committee and an informed consent was obtained from all the study subjects. The data obtained were analyzed using SPSS software 22.0. All categorical variables were analyzed using chi-square test and all continuous variables were analyzed using students t-test or other appropriate tests. P-value<0.05 was considered as statistically significant.

Figure 1: Study Protocol
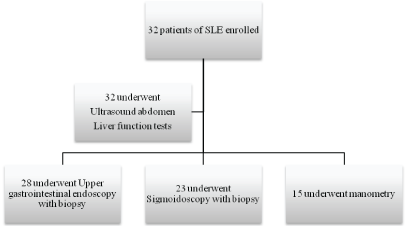
Figure 2: Study design
The study included 32 SLE patients, 2 males (6.2%) and 30 females (93.8%). The mean age of the patients was 26.22 ± 7.77 years (range of 17 to 64 years). The mean duration of the illness was 2.88 ± 2.49 years.SLE was diagnosed on basis of modified ACR criteria, all patients fulfilled ≥ 4 criteria, of these 16 (50%) patients met five of eleven criteria, 11 (34.3%) patients met four criteria, 2 (6.2%) patients each fulfilled six and eight criteria, 1 (3.1%) patient fulfilled seven criteria. 7 patients had neurological manifestation (2 mononeuritis multiplexa and 5 psychosis), 4 patients had cardiovascular involvement (3 pericardial effusion, 1 pulmonary hypertension), 9 patients had renal involvement (6 class IV LN and 3 Class V LN), 9 patients had endocrine involvement (all were hypothyroid, 2 were diagnosed as primary hypothyroidism for the first time), haematological involvement seen in 30 patients (in that 3 autoimmune haemolytic anemia and 2 immune thrombocytopenia). Liver function tests were done, mean ofserum protein 5.27+0.64 mg/dL (4.2 to 6.9 mg/dL), serum albumin 3.67 ± 0.55 mg/dL (2.8 to 4.7 mg/dL), SGOT 18.13 ± 9.75 mg/dL (10 to 65 mg/ dL), SGPT 19.81 ± 10.17 mg/dL (12 to 70 mg/dL) and serum ALP 8.10 + 6.12 (3 to 40 IU/dL). 30 (93.75%) patients had symptoms suggestive of gastrointestinal involvement (Table 1). The commonest symptom was oral ulcer (84.4%, n=27). Other symptoms were heartburn (40.6%, n=13), epigastric pain (15.6%, n=5), dry mouth (9.4%, n=3), dyspepsia(6.3%, n=2), constipation (10%, n=3), dysphagia (6.3%, n=2), odynophagia (3.1%, n=1), anorexia (3.1%, n=1), diarrhoea (3.1%, n=1), jaundice (3.1%, n=1) and ascitis (3.1%, n=1).There was statistically significant co-relation between gastrointestinal and neurological, endocrine involvement (p-0.04 and 0.03 respectively) as indicated in table 2.
| Symptoms | All pts. (n=32) | n (%) |
| Oral ulcer | 27 | 84.4% |
| Heart burn | 13 | 40.6% |
| Epigastric pain | 5 | 15.6% |
| Dry mouth | 3 | 9.4% |
| Dyspepsia | 3 | 9.4% |
| Constipation | 3 | 9.4% |
| Dysphagia | 2 | 6.3% |
| Odynophagia | 1 | 3.1% |
| Anorexia | 1 | 3.1% |
| Diarrhea | 1 | 3.1% |
| Ascitis | 1 | 3.1% |
| Liver dysfunction | 1 | 3.1% |
Table 1: Distribution of gastrointestinal symptoms
| With gastrointestinal involvement (n=23) | Without gastrointestinal involvement (n=9) | p-value | |
| Neurological involvement | 7 | 0 | 0.04 |
| Cardiovascular involvement | 4 | 0 | 0.16 |
| Pulmonary involvement | 1 | 0 | 0.50 |
| Renal involvement | 8 | 2 | 0.491 |
| Endocrinal involvement | 7 | 0 | 0.027 |
Table 2: Correlation between gastrointestinal involvement and other organ involvement
Gastrointestinal and hepatic symptoms were scored based on presence of symptom, severity and duration of symptom. The presence of symptom was given a score of 1, severity graded as per the symptoms into 4 grades and scored; similarly the duration was scored. Out of 32 symptomatic patients, 15 had more than 1 symptom and 2 were asymptomatic.
All patients underwent ultrasonologic examination, 28 patients agreed for upper gastrointestinal endoscopy with biopsy, 23 patients underwent sigmoidoscopy with biopsy and 15 patients underwent manometry. The rest did not consent for the procedure. On endoscopy, the prominent finding seen was oral ulcers, seen in 25 patients, glossitis or fissuring of tongue was seen in 12 patients and angular stomatitis in 3 patients. Abnormal findings were seen in 15 of 28 patients (53.3%) who underwent upper gastrointestinal endoscopy (Figure 3). The pattern of abnormality were, hyperaemia in lower end of esophagus in 6, mild gastritis in 11, atrophic gastritis in 4, antral gastritis in 9 patients. Sigmoidoscopy was normal in all patients who underwent the procedure, Ultrasonography revealed fatty liver in 2 (6.9%) patients and hepatomegaly with normal echotexture in 2 (6.9%) patients.
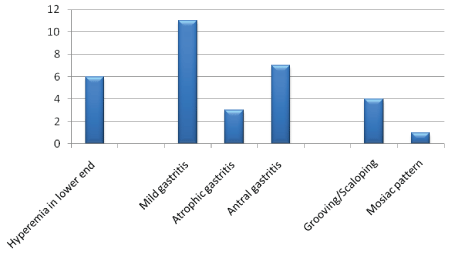
Figure 3: Pattern of Upper gastrointestinal endoscopy findings.
On esophagealmanometry, 4 out of 15patients (26.67%) showed abnormal esophageal motility. Hypertensive LES with nutcracker esophagusin 1 patient, Nutcracker esophagus in 3 patients, hypotensive LES in 1 patient. The LES with swallow was abnormal in 4(13.33%) and mean basal LES pressure was 12.20 ± 2.74. Histopathology of the gastrointestinal mucosa was abnormal in 19 patients (66.6%). 11 (39.3%) patients had atrophic gastritis and 8 (28.6%) patients had chronic inflammatory infiltrates. Of these six, 3 (10.7%) patients had H.pylori infection and 5(17.8%) patients had no specific etiology. Duodenal biopsy was abnormal (non specific inflammation) in 7 patients. There were no other findings suggestive of any vasculitis or dysplasia. Sigmoid mucosal histology was abnormal in 9(27.2%) patients. Of these 7(13.4%) had collagenous colitis. There was no statistically significant relationship between abnormal manometric findings and oesophageal symptoms.
Gastrointestinal symptom scoring had significant correlation with histopathology of GI tract after excluding oral symptoms. When GI symptom scoring compared with UGI endoscopy, scoring ≥ 8 showed significant correlation with abnormal UGI endoscopy and histopathology (Table 3).
| UGIE abnormal | UGIE normal | P | |
| Score ≥ 12 | 6 | 1 | 0.000(ᵞ) |
| Score ≥ 8 | 4 | 0 | 0.004(ᵞ) |
| Oesophageal symptoms | 9 | 3 | 0.049 |
| Stomach symptoms | 3 | 4 | 0.512 |
Table 3: Comparison of symptom scoring with UGI endoscopic abnormality
UGIE- Upper gastrointestinal endoscopy
The patients were grouped into 2 groups: Group-1 with gastrointestinal involvement based on the evidence of abnormal manometry, histopathological involvement of gastrointestinal tract and group-2 had no gastrointestinal involvement. Only the level of hemoglobin showed p<0.05 stating there was statistical significant difference between them (Table 4).The clinical symptomatology was scored in all the patients and the histological findings of gastrointestinal tract were noted. These findings were ranked on a simple scale and converted to ordinal variables. Histological findings were grouped as, 0=not done, 1=nonspecific changes, 2=disease specific changes (i.e., changes seen in systemic lupus erythematosus)
| With GI involvement (n=23) | Without GI involvement (n=9) | p-value (t-test) | |
| Mean age | 25.87 ± 7.71yrs | 27.11 ± 8.32 yrs | 0.53 |
| Mean duration | 2.74 ± 2.68 yrs | 3.22 ± 2.04 yrs | 0.32 |
| Hemoglobin | 9.87 ± 1.08 g % | 9.06 ± 2.20 g % | 0.04 |
| Serum protein | 5.34 ± 0.54 mg% | 5.08 ± 0.68 mg% | 0.56 |
| Serum albumin | 3.69 ± 0.57 mg% | 3.64 ± 0.51 mg% | 0.63 |
| Basal LES | 23.43 ± 3.57 mmHg | 23 ± 1 mmHg | 0.42 |
| Wave progression | 3.18 ± 0.14 cm/s | 2.90 ± 0.3 cm/s | 0.52 |
| Distal wave amplitude at 3cm | 49.07 ± 1.69 mmHg | 46 ± 1 mmHg | 0.10 |
| Distal wave amplitude at 8cm | 72.43 ± 2.22 mmHg | 76 ± 8 mmHg | 0.67 |
Table 4: Comparison of various parameters in patients with and without gastrointestinal manifestation
These scores were later co-related like on the regular Pearson productmoment correlation coefficient by Spearman ρ. Results obtained showed that there was no correlation between gastrointestinal symptomatology scoring and histological findings of gastrointestinal tract (With correlation co-efficient=0.357, ρ =0.053). It was also noted once the oral cavity symptoms were excluded from the gastrointestinal symptom scoring there was significant correlation with the histopathological findings (With correlation co-efficient=0.366, ρ =0.047).
One of the most disturbing and at times debilitating, manifestations of lupus involves the gastrointestinal tract. This can result in significant patient discomfort and complications. Due to the organ systems which comprise the GI tract, these manifestations can take one of several forms.
In our series 30 (93.75%) patients had gastrointestinal manifestations. In available literature, the prevalence varies from 25 to 40% [3]. The high prevalence noted in our series is probably due to inclusion of relatively non-specific features such as oral ulcers and dyspepsia. Usually, patients with lupus will seek medical attention because of classic manifestations of lupus such as arthralgias and rash. In reviewing case studies, GI manifestations of lupus as the initial presentation rather than other more classic symptoms occur in approximately l-5% of patients with lupus [3,4]. None of the patients in our study had gastrointestinal symptoms as their initial manifestation.
Data on endoscopic findings is sparse perhaps intrinsic to its invasive nature. Sultan et al. [3] reported oesophagitis in 3-5% of cases. Ginzler et al. [5] found gastric involvement in 10-15%. 15 out of 28 patients (53.3%) in our series showed abnormal upper gastrointestinal endoscopy. Sigmoidoscopy was normal in all 23 patients who agreed for the procedure. Organ involvement in lupus can include the esophagus, stomach, small intestines, colon, pancreas, peritoneum and liver. Microscopic examination of these tissues reveals the presence of an arteritis involving small blood vessels which is similar to the pathologic findings in other organ systems. Thus the GI manifestations of lupus are dependent upon an arteritis involving that particular GI organ. This organ involvement then results in the manifestations of nausea, vomiting, abdominal pain or diarrhea. In association with the arteritis and intense inflammatory activity, ischemia and ulcerations can occur in the involved organ, resulting in further complications. 13 patients (43.33%) had abnormal histology. The commonest finding was atrophic gastritis. Gastric ulcer was detected in 4-21% of patients in a study by Saab et al. [6] Most of their patients were on NSAIDs or corticosteroids. Medina et al. [8] reported perforated peptic ulcer in 6% of their patients while Al Hakeem et al. [9] had 8% with similar complication. We did not encounter a perforation in any of our subjects.
In lupus, due to involvement of esophagus, patients can have difficulty with initiation of swallowing or develop painful swallowing. These symptoms are usually mild and occur most often with solids rather than liquids. The basis of these complaints can be secondary to a motility (muscular disorder) or severe arteritis resulting in inflammation and ulceration of the esophagus. Motility studies performed in SLE reveal usually mild to marked motility disturbances in the esophagus resulting in dysphagia. These motility findings are separate and distinct from findings present in patients with other auto-immune disorders, such as scleroderma. At times, a patient with lupus will develop a sclerodermalike picture with involvement of the esophagus along with telangiectasias and Raynaud’s phenomenon which may make differentiation from true scleroderma extremely difficult. Manometry studies reveal functional abnormalities of the esophagus in 10-32% of patients with SLE. [10,11] Aperistalsis or hypoperistalsis is most frequently found in the upper one-third of the esophagus [12] the lower esophageal sphincter was less commonly affected. In our study abnormal manometry was seen in 4 patients with 3 patients had nutcracker esophagus, 1 had hypertensive lower esophageal sphincter (LES) and none of them had hypomotility. Other complicating factors associated with esophageal involvement are concurrent medications such as steroids or azathioprine or cyclophosphamide or mycophenolate which result in suppression of the patient’s immune system. In this setting, fungal or herpes infection of the esophagus can occur, resulting in symptoms of difficulty swallowing.
Other upper abdominal complications of lupus include nausea and vomiting which may result from arteritis involving the stomach or small intestine or a so-called pseudo-obstruction of the intestine. This pseudo-obstruction most likely represents a motility disturbance of the small intestine related to lupus arteritis. The importance of this entity is to distinguish this from a true obstructive process which would require surgical intervention for relief of the obstruction. Another organ in the GI tract less often involved in lupus is the pancreas. Patients with lupus can develop acute and chronic pancreatitis secondary to arteritis involving focal areas of the gland or the gland exclusively. Symptoms are those of recurrent nausea and vomiting which must be distinguished from pseudoobstruction. This entity usually is diagnosed by a symptom complex and laboratory studies demonstrating acute inflammation of the pancreas. Fortunately enough, the pancreas is not often involved to this extent in most patients with lupus. At times, however, the pancreatitis itself can be secondary to concomitant medications used in the treatment of lupus such as immunosuppressive and steroids. Treatment, however, remains the same in both of these entities. Pancreatitis occurs in as many as 2 to 8 percent of patients, usually in those with active SLE elsewhere. We did not encounter pancreatitis in our observation [13].
Involvement of the large intestine or colon also occurs with some frequency in GI lupus. Manifestations include diarrhea, lower abdominal discomfort, pain related to the arteritis, and at times, ulceration of the large intestine. To diagnose this entity requires x-ray or endoscopic visualization of the colon to rule out other pathologic processes. Treatment is, again, of the underlying disease with non-steroidal and anti-inflammatory agents in an attempt to improve the arteritis involving the large intestines. The prevalence of intestinal vasculitis in patients with SLE ranged from 0.2 to 1.1% in recent studies [14]. None of our subjects had this complication.
Hepatomegaly can occur but is usually mild and only at times discomforting to patients. Frequently, routine biochemical profiles will show a mild hepatitis (inflammation of the liver) although no clinical symptoms to suggest hepatitis. This hepatitis is frequently due to concomitant medications such as aspirin or a mild arteritis involving the liver. It is important to be aware of this entity, because lupus involvement of the liver is usually mild and it does not progress to severe hepatitis or cirrhosis. Runyon et al. [15] diagnosed significant liver disease in 43 patients (21%). Gibson and Myers [16] studied 81 patients with SLE and reported that 45 (55%) had abnormal liver function results at some point. One patient in our series had jaundice, 3 had hepatomegaly and 2 others had fatty infiltration in the liver.
There was correlation between the neurological and endocrine involvement with gastrointestinal tract involvement. Neurological involvement was seen in 7 patients of them 2 had mononeuritis multiplex and 5 hadpsychosis. Endocrine involvement was seen in 9 patients as hypothyroidism. There was no correlation between clinical gastrointestinal symptom scoring and histological findings of gastrointestinal tract. It was also noted once the oral cavity symptoms were excluded from the gastrointestinal symptom scoring there was significant correlation with the histopathological findings. A larger study population is necessary to draw a meaningful conclusion and to find a definite correlation between the various parameters.
Systemic lupus erythematosus may present with protean GI manifestations. Fortunately, majority of them are mild. The underlying process is much similar to other organ involvement, their exist a significant co-relation between GI symptoms and histopathological findings, with appropriate treatment, these manifestations can be significantly improved to allow a more normal life-style.
Download Provisional PDF Here
Article Type: Research Article
Citation: Gupta KL, Pattanashetti N, Babu J, Dutta U (2017) Study of Gastrointestinal and Hepatic Manifestations in Systemic Lupus Erythematosus. Int J Nephrol Kidney Failure 3(2): doi http://dx.doi.org/10.16966/2380-5498.147
Copyright: © 2017 Gupta KL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access