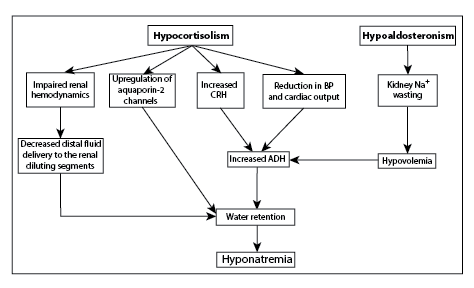
Figure 1: Pathogenetic mechanism of hyponatremia in primary adrenal insufficiency.
CRH: Corticotropin-Releasing Hormone; BP: Blood Pressure; ADH: Antidiuretic Hormone

Filippatos TD Liamis G Liontos A Elisaf MS*
Department of Internal Medicine, School of Medicine, University of Ioannina, Ioannina, Greece*Corresponding author: Moses S Elisaf, Professor, Department of Internal Medicine, School of Medicine, University of Ioannina, Ioannina, Greece, Tel: +302651007509; Fax: +302651007016; E-mail: egepi@cc.uoi.gr, melisaf54@gmail.com
Primary adrenal insufficiency (Addison’s disease) is a rather common etiology of decreased sodium levels (serum sodium <135 mmol/L). Aim of the short review is to present the current evidence of the pathogenetic mechanisms, diagnosis and treatment of hyponatremia in patients with primary adrenal insufficiency. The pathogenesis of hyponatremia in patients with primary adrenal insufficiency is mainly attributed to increased secretion of antidiuretic hormone (ADH). Certain clues may aid clinicians to differentiate primary adrenal insufficiency and the syndrome of inappropriate ADH secretion (SIADH). Treatment of hyponatremia in patients with primary adrenal insufficiency includes cortisol administration and volume repletion. Clinicians should be vigilant for the exclusion of primary adrenal insufficiency in patients with hyponatremia.
Hyponatremia; Sodium; Primary adrenal insufficiency; Syndrome of inappropriate antidiuretic hormone secretion; Cortisol
Primary adrenal insufficiency (Addison’s disease), which is usually caused by autoimmune adrenalitis (often associated with autoimmune disorders) and less frequently by infections (e.g. tuberculosis, human immunodeficiency virus, fungal infections) metastatic carcinoma, hemorrhage and medications, is a rather common etiology of decreased sodium levels (serum sodium <135 mmol/L) [1-30]. However, the true incidence of primary adrenal insufficiency in patients with hyponatremia is not clear, since evidence from prospective and retrospective clinical trials indicates that basal serum cortisol levels are not measured routinely even in patients with unexplained decreased serum sodium levels. Moreover, despite the association between adrenal insufficiency and hyponatremia, a more detailed investigation of the hypothalamic, pituitary and adrenal function is rarely performed [31-37].
The pathogenesis of hyponatremia in patients with primary adrenal insufficiency is mainly attributed to increased secretion of antidiuretic hormone (ADH), which in turn results in water retention and dilutional decrease of serum sodium levels. The increased secretion of ADH in this case is caused by cortisol deficiency, which is a physiologic tonic inhibitor of ADH secretion and exerts negative feedback on corticotropin-releasing hormone (CRH) and ADH; hence, cortisol deficiency leads to increased CRH and ADH secretion [38-41]. Additionally, ADH is secreted in parallel with CRH by the paraventricular nuclei cells in the hypothalamus, since it is an important ACTH secretagogue [42]. The increased ADH secretion in patients with adrenal insufficiency is also attributed to alterations in systemic hemodynamics (reduction of blood pressure and cardiac output), which in turn stimulate the baroreceptor-mediated ADH secretion [28,40,41,43]. Finally, it has been shown that the administration of glucocorticoids can directly suppress pituitary ADH secretion and their discontinuation may be followed by an increased secretion of ADH [44- 48] (Figure 1).

Figure 1: Pathogenetic mechanism of hyponatremia in primary adrenal insufficiency.
CRH: Corticotropin-Releasing Hormone; BP: Blood Pressure; ADH: Antidiuretic Hormone
Experimental data support the etiological correlation of increased ADH secretion and hyponatremia in primary adrenal insufficiency. The administration of vaptans, which act as ADH V2 receptor antagonists in the kidney, normalizes the urinary dilution ability in adrenalectomized mineralcorticoid–replaced rats [49]. Additionally, an increase in renal sensitivity to ADH, shown as up-regulation of renal aquaporin-2 water channels, is implicated in the development of hyponatremia in glucocorticoid-resistant rats [50].
It is also possible that ADH-independent mechanisms may play a role in the pathogenesis of hyponatremia in primary adrenal insufficiency. In this context, factors such as impaired renal hemodynamics and decreased distal fluid delivery to the diluting segments of the nephron may provoke hyponatremia [41,45]. Experimental data supports this concept, since it has been shown that glucocorticoid deficiency is associated with upregulation of the Na+-K+-2Cl- co-transporter, the Na+-H+ exchanger isoform 3 and the cortical β and γ subunits of epithelial sodium channel, which promotes sodium retention and reduces fluid delivery to the distal nephron diluting segments and finally results in decreased free-water excretion. However, this ADH-independent mechanism on water excretion capacity becomes significant only in cases of increased water intake [49].
Primary adrenal insufficiency also results in aldosterone deficiency, which significantly contributes to the pathogenesis of hyponatremia. Aldosterone deficiency promotes renal sodium wasting, hypovolemia and baroreceptor-mediated increased secretion of ADH [3,6,51,52]. Thus, aldosterone deficiency causes hyponatremia through two mechanisms: i) the increased levels of ADH and subsequent upregulation of aquaporin-2 water channels in the renal tubules, and ii) the extracellular volume depletion-induced reduction in GFR inducing increased proximal tubular sodium reabsorption and reduced fluid delivery to the distal diluting segment of the nephron [38,53].
In some cases of autoimmune polyendocrinopathy, primary adrenal insufficiency is accompanied by hypothyroidism that, if severe and especially if causing myxedema, may contribute to the pathogenesis of hyponatremia [54].
It should be mentioned that the most causes of primary adrenal insufficiency impair the adrenal cortex as a whole and may result in concurrent deficiencies of cortisol, aldosterone and adrenal androgen. Thus, acute adrenocortical insufficiency (adrenal crisis) is mandatory to be included in the differential diagnosis of a hyponatremic patient who has weakness, abdominal pain, nausea, vomiting, diarrhea, fever, lethargy, confusion or coma [28]. Chronic adrenocortical insufficiency (Addison’s disease) is usually characterized by the pattern of hyponatremia, hyperkalemia and hypovolemia (Table 1). Certain clinical (hypotension, skin hyperpigmentation of the sun-exposed surfaces or even oral mucosa) and laboratory findings (hyperkalemia, raised blood urea, hypoglycemia, hypercalcemia, eosinophilia) may aid the diagnosis.
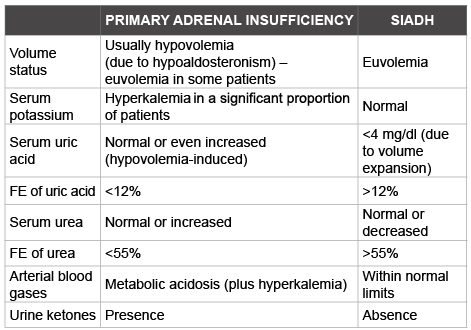
Table 1: Differential diagnosis between primary adrenal insufficiency and syndrome of inappropriate antidiuretic hormone secretion (SIADH) FE: Fractional Excretion
However, in some case the diagnosis of Addison’s disease is difficult, since primary adrenal insufficiency can present without hypovolemia, orthostatic hypotension or hyperkalemia. For example, the presence of hyponatremia in patients with hypoaldosteronism and renal salt wasting may cause red blood swelling resulting in increased plasma volume and elevated interstitial pressure that promotes shift of water from the interstitial to the intravascular volume. Additionally, the hypovolemiainduced increased symphathetic nervous system activity promotes venous vasoconstriction and subsequently reduces the volume of the vascular compartment leading to increase of filling pressures [55-57].
Furthermore, hyperkalemia is evident only in approximately 50- 60% of patients with primary adrenal insufficiency [58,59]. The absence of hyperkalemia may be due to isolated hypocortisolism (for example due to autoantibodies with a high affinity for the zona fasciculata) or to aldosterone-independent regulation of potassium metabolism (for example decreased potassium intake or extrarenal potassium losses). Additionally, patients with autoimmune disorders or cancer may have circulating cation proteins that activate the calcium sensing receptor in the thick ascending limb of Henle, resulting in inhibition of the K+-Na+- Cl- cotransporter and subsequently promoting natriuresis and kaliuresis [29,55,60-62].
Patients with primary adrenal insufficiency without significant hypoaldosteronism may have euvolemic hyponatremia with a ‘SIADHlike picture that is characterized by increased urinary osmolality and urine sodium concentration [63]. SIADH is one of the most common causes of hyponatremia in the clinical practice. Its diagnosis is based on clinicolaboratory criteria after the exclusion of other commonly causes of hyponatremia, including thiazides administration, salt-wasting nephropathy, cancer, and endocrinopathies, such as primary and secondary adrenal insufficiency and severe hypothyroidism [6,64-72]. In patients with a ‘SIADH-like’ picture, certain clues may aid clinicians to differentiate between hyponatremia due to primary adrenal insufficiency and hyponatremia due to SIADH (Table 1) [29]. These differences are mainly attributed to the fact that primary adrenal insufficiency is associated with hypoaldosteronism-related hypovolemia causing low fractional excretion rate of uric acid and urea, whereas SIADH is associated with high values of these parameters [1,69,73]. Additionally, a useful and easy test to differentiate between SIADH and adrenal insufficiency is the presence of ketonuria, which is detected in patients with hyponatremia associated with adrenal insufficiency due to increased fatty acid oxidation and subsequent production of ketones [29].
The diagnosis of primary adrenal insufficiency is established if ACTH levels are elevated (>2-fold the upper limit of the reference range) and in the standard dose ACTH stimulation test (250 µg corticotropin iv) serum cortisol concentrations 30-60 minutes after the administration of synthetic ACTH are <18 µg/dl (<500 nmol/L) [74]. Patients taking glucocorticoid supplements or spironolactone should not take them on the day of the test. If the ACTH stimulation test is not available, the diagnosis of adrenal insufficiency is based on serum cortisol and ACTH levels, which should be measured at 8 to 9 am to avoid influences of their circadian rhythm. Elevated ACTH levels (>2-fold the upper limit of the reference range) with low cortisol [<5 µg/dL (<140 nmol/L)] are highly suggestive, especially in patients who are in stress. Even ACTH levels within the normal range are rather inappropriate when cortisol levels are very low. The simultaneous measurement of plasma renin and aldosterone to determine mineralcorticoid deficiency is also recommended [74].
Cortisol administration and volume repletion result in decrease of ADH secretion and increase of serum sodium levels in hyponatremic patients with primary adrenal insufficiency. It should be mentioned that volume repletion should be established with careful administration of isotonic saline solutions and with repeated measurements of serum sodium levels in order to prevent inappropriate overcorrection of serum sodium concentration that may result in the osmotic demyelination syndrome with its devastating consequences [75-80]. Additionally, mineralocorticoid replacement is needed especially in patients who have primary adrenal insufficiency with clinical or laboratory findings suggesting hypovolemia. In these cases careful monitoring and adjustment of treatment are mandatory [10,74,81].
It should be mentioned that in patients who have both primary adrenal insufficiency and hypothyroidism, hydrocortisone replacement should be given first, before thyroid hormone replacement, because treatment of hypothyroidism in patients with untreated adrenal insufficiency may result in addisonian crisis due to profound reduction of circulating cortisol levels owing to thyroxin-induced cortisol clearance and to increased cortisol requirements owing to thyroxin-induced restoration of basal metabolic rate [82].
Download Provisional PDF Here
Article Type: Short Communication
Citation: Filippatos TD, Liamis G, Liontos A, Elisaf MS (2016) Hyponatremia in Primary Adrenal Insufficiency: An Often Overlooked Cause of Decreased Sodium Levels. Int J Nephrol Kidney Failure 2(3): doi http://dx.doi.org/10.16966/2380-5498.130
Copyright: © 2016 Filippatos TD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access