Abstract
Background: Hyperuricemia and obesity are associated with more severe renal pathology and more progressive disease in adults with
IgA nephropathy (IgAN). However, only one study has described the prevalence of hyperuricemia and none has described obesity in pediatric
patients with IgAN.
Methods: The prevalence of hyperuricemia and obesity in IgAN patients 7 to 18 years of age was compared between multiple centers in the
USA and Canada (USA/CAN group) and 2 centers in the Czech Republic and Germany (CZE/GER group). Serum uric acid (SUA) and estimated
glomerular filtration rate were measured in 79 USA/CAN patients and 48 CZE/GER patients. Body mass index percentiles (BMI percentiles) were
compared between 51 of the 79 USA/CAN patients and all 48 CZE/GER patients.
Results: The prevalence’s of both hyperuricemia and obesity were higher in the USA/CAN patients than the CZE/GER patients: 35.4% versus
16.7%; p=0.026, and 35.3% versus 4.2%, p<0.001, respectively. The BMI percentile in the combined populations was significantly higher in
hyperuricemic patients (78.9 ± 21.6 versus 60.2 ± 31.0), p=0.003.
Conclusions: There is a much higher prevalence of hyperuricemia and obesity in pediatric IgAN patients in the USA/CAN compared to CZE/
GER. These abnormalities may have serious long-term consequences for the USA/CAN patients.
Keywords
BMI percentile; Hyperuricemia; IgA Nephropathy; Obesity
Introduction
Hyperuricemia (HU) is considered by some investigators to be an
independent risk factor for both cardiovascular and cerebrovascular events
and for progressive disease in patients with chronic kidney disease (CKD),
and is also associated with obesity, metabolic syndrome, albuminuria
and essential hypertension, even in children [1-6]. In studies conducted
around the world, HU has been detected in up to 51.5% of adult patients
with IgA nephropathy (IgAN). This combination is often associated with
more severe renal histopathology and a poor prognosis [7-10]. Only one
report from Seeman et al. [11] has described the prevalence of HU in
children and adolescents with IgAN. They reported a low prevalence of
HU (14%) in their patients. Obesity has also been incriminated as a risk
factor in adult patients with IgAN [12,13]. Although there are no reports
of obesity in children with IgAN, severe obesity was shown recently to be
associated with an increased prevalence of cardio metabolic risk factors in
children and adolescents 3-19 years of age in the USA [14].
In this observational study, we compare serum uric acid (SUA) levels
in 79 IgAN patients ≥ 7 to ≤18 years of age in the USA and Canada
(USA/CAN) with 48 patients of comparable age in the Czech Republic
and Germany (CZE/GER). Some of the CZE/GER patients were included
in the previous report referenced above [11]. We also compare the
prevalence rates of HU in patients of comparable age based on samples
drawn up to 20 years apart in each of the locations. In addition, we
compare the prevalence of overweight and obesity (based on body mass
index percentiles (BMI percentiles)) in the USA/CAN and CZE/GER
patients. Finally, we examine the correlations between SUA and CKD
Stage (1 versus 2), obesity, blood pressure levels, and proteinuria.
Methods
Objectives of the study
1) To compare the prevalence of HU in children and adolescents
with IgAN and relatively well preserved renal function (eGFR ≥ 60 ml/
min/1.73 m2
) in the USA/CAN with those in CZE/GER; 2) to correlate
these SUA measurements with CKD Stage 1 versus 2; and 3) compare the
prevalence of obesity in the USA/CAN versus CZE/GER and evaluate the
relationship between HU and obesity in the two populations.
Inclusion criteria: 1) age ≥ 7 to ≤ 18 years; 2) renal biopsy diagnostic
for IgAN; 3) SUA and serum creatinine drawn simultaneously on at least
one occasion, and 4) CKD Stages 1 (eGFR ≥ 90 ml/min/1.73m2
) or 2
(eGFR ≥ 60-89 ml/min/1.73 m2
).
Exclusion criteria: 1) systemic lupus erythematosus, 2) HenochSchönlein
purpura (HSP), 3) chronic liver disease or hepatitis, 4) CKD
stages 3-5, 5) use of allopurinol or other medications that were given to
reduce SUA levels.
Clinical Evaluations
Height, weight and blood pressure (BP) were measured and BMI
percentiles were determined using the Centers for Disease Control and
Prevention (CDC) on-line Calculator (http://nccd.cdc.gov/dnpabmi/
Calculator.aspx).
Laboratory Evaluations
In the USA/CAN patients, SUA and serum creatinine (SCr)
measurements were obtained as part of studies performed to determine
eligibility for one of two prospective randomized clinical trials.
Measurements in the CZE/GER patients were carried out as part of
routine nephrologic studies in the clinical laboratories of the participating
institutions. SUA concentrations were measured using an enzymatic
colorimetric test. SCr was measured with a kinetic colorimetric assay
(Jaffé method). SCr levels were used to calculate eGFR and CKD Stages
according to the K/DOQI guidelines described in [15].
Definitions
For consistency, we will use the definitions for HU that were used
previously by Seeman et al. [11], i.e. HU= SUA>5.88 mg/dl (>350 µmol/L)
in all girls and in boys less than 15 years of age; and >7.06 mg/dl (>420
μ mol/L) in boys 15-18 years of age. Prevalence of HU was determined
a) in 2 time periods; b) in boys and girls; and c) in two age categories:
<15 years of age and 15-18 years of age. Persistent HU is defined as
consistent elevation of SUA measurements (≥ 2 in each patient), which
were available in most of the patients. Obesity is defined as BMI ≥ 95th
percentile; overweight is defined as BMI ≥ 85 < 95th percentile [14].
Concomitant medications taken by patients when SUA levels
were drawn
The medication class that was most frequently given to patients in
both populations was an angiotensin-converting enzyme inhibitor
(ACEi) (USA/CAN group: 39 of 78 patients (50%); CZE/GER group: 17
of 47 patients (36.2%); overall: 56 of 125 patients (44.8%); ACEi status
was missing in 2 patients. The indications for starting an ACEi varied,
i.e. hypertension versus proteinuria. Hence, we cannot conclude that
patients on ACEi were hypertensive when the ACEi was started. Less
than 10% of the patients were on other medications: fish oil: 7 patients,
corticosteroids: 6 patients, thiazides: 5 patients, and angiotensin
receptor blockers: 5 patients.
Statistics
Data were abstracted from clinical and study records and imported
into SPSS ver. 22 (IBM Corp, Armonk NY). Continuous data are reported
as means (SD); categorical data as counts (%). Analysis of variance or
Students t-tests were used to analyze continuous data; chi-square analysis
or Fisher’s exact tests were used for categorical data. Pearson correlation
coefficients were used to estimate the strength of the relationship between
variables. An alpha of 0.05, two-tailed, was set as the criterion for statistical
significance.
Results
Patient population
One hundred and twenty-seven patients fulfilled the eligibility criteria
(79 from USA/CAN and 48 from CZE/GER). Clinical and laboratory
features are shown in table 1. There were no differences in age, sex, height,
serum creatinine or BP between the two groups. The mean body weight,
BMI and BMI percentiles in the USA/CAN patients were significantly
greater than those in the CZE/GER patients (p<0.001 for both). The mean
eGFR and percentage of CKD 1 patients were lower in the CZE/GER
patients but the majority of both populations had CKD 1.
SUA levels and HU
Whereas the initial SUA levels in the USA/CAN and CZE/GER patients
were not statistically different (p=0.068), the percentage of patients with
HU based on these initial blood samples was significantly greater in the
USA/CAN group than in the CZE/GER group (35.4% versus 16.7%),
p=0.026) and serial SUA measurements were considerably higher in the
USA/CAN patients. Overall, 2 or 3 SUA measurements were obtained
in 61 USA/CAN patients and 25 CZE/GER patients. Persistent HU was
present in 42.6% of the USA/CAN patients and 12% of the CZE/GER
patients. The mean SUA based on 2-3 measurements was 6.01 ± 1.88 mg/
dl in the USA/CAN group and 4.80 ± 1.40 mg/dl in the CZE/GER group,
p=0.005 (Table 1).
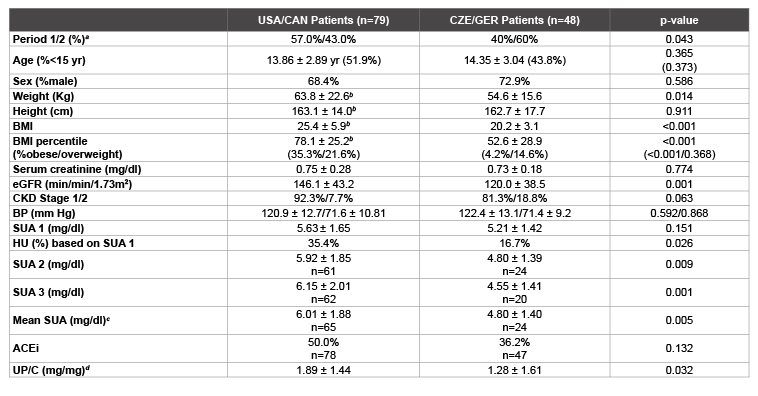
Table 1: Clinical and laboratory features in patients with IgAN from USA/CAN and CZE/GER
a
Period 1 = pre 2003; period 2 = 2003 and beyond
b
Height, BMI and BMI percentile available in only 51 of the USA/CAN patients. Body weight was 62.2 ± 20.5 kg and
serum creatinine was 0.76 ± 0.25 mg/dl in the 51 patients.
c
Mean SUA based on 2 or 3 measurements of SUA in each patient .
d
UP/C (=urine protein to creatinine) ratios calculated from first morning or 24-hour urines. Urine creatinines in CZE/
GER patients were divided by 88.4 to convert the results from mg/mmol to mg/dl.
Subsequent analyses in the 2 patient populations showed that the
prevalence of HU in children and adolescents varied according to sex,
location and time period (Table 2). In period 1, the prevalence of HU in
the USA/CAN group (1996-1999) versus the CZE/GER group was not
significantly different (28.9% versus 15.8%, p=0.353). However, in period
2 (2003-2006), the prevalence of HU was greater in the USA/CAN group
than the CZE/GER group (2003-2014) (44.1% versus 16.1%, p=0.030),
and also when the two time periods were combined (35.4% versus 16.3%,
p=0.026).The increase in SUA from period 1 to period 2 was similar in the
2 locations (USA/CAN: +0.59 (5.18 up to 5.77) mg/dl., CZE/GER: +0.67
(4.81 up to 5.48) mg/dl.).
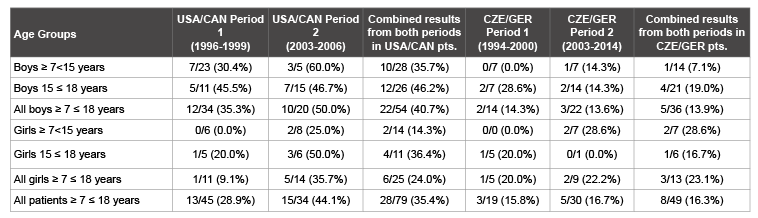
Table 2: Prevalence of hyperuricemia (HU)a
in children with IgAN and CKD 1 /2 (i.e. eGFR ≥ 60 ml/min/1.73m2 )
a
HU defined as SUA >5.88 mg/dl(>350 μ Mol/L) in all girls and boys ≥ 7<15 years of age; >7.06 mg/dl (>420 μ Mol/L) in boys 15 ≤ 18 years of age.
Prevalence of obesity and overweight
There was a very high prevalence of obesity (35.3%) and overweight
(21.6%) in the 51 USA/CAN patients (prevalence of obesity and overweight
combined was 56.9%). The prevalence of obesity and overweight in the
CZE/GER patients was 4.1% and 14.6% respectively (18.7% combined).
Hence, there was an 8.6 fold higher prevalence of obesity and a 3 fold
higher prevalence of obesity/overweight combined in the USA/CAN
patients, p<0.001. There was no significant difference in the prevalence of
obesity in the USA/CAN group over time, but in the CZE/GER patients,
there was a higher prevalence of obesity and overweight combined in the
second period (29%) compared to the first period (0%), when the highest
BMI percentile among the 18 patients was only 74%.
Relationship between BMI percentile and SUA levels
The BMI percentile was significantly greater in the 30 hyperuricemic
patients (78.9 ± 21.6) than the 69 normouricemic patients (60.2 ± 31.0,
p=0.001) when the USA/CAN and CZE/GER patients were combined. The
correlation between BMI percentile and SUA was statistically significant,
r=0.245, p=0.014. The overall prevalence of HU was significantly greater
in the obese/overweight children than in the non-overweight/non-obese
children, i.e. 43.2% (16/37) versus 21.0% (13/62), p=0.015.The mean
SUA was significantly higher in obese/overweight children versus nonoverweight/non-obese
children 6.10 ± 1.38 versus 5.35 ± 1.58, p=0.018.
When data from the 2 locations were evaluated separately, HU was
present in 12.5% of the CZE/GER patients with BMI% < 85 versus 33.3%
of the patients with BMI percentile ≥ 85; HU was seen in 36.4% of the
USA/CAN patients with BMI percentile < 85 versus 48.3% of those with
BMI percentile ≥ 85.
Relationship between CKD stages and SUA levels
CKD Stage 1 was present in 73/79 USA/CAN patients and 39/48 CZE/
GER patients. CKD Stage 2 was present in 6 USA/CAN patients and 9
CZE/GER patients. The overall prevalence of CKD Stage 1 in the two
populations was therefore 112 of 127 (88.1%) and CKD Stage 2, 11.9%.
The prevalence of HU in CKD Stage 1 patients (27.0%) did not differ from
that of CKD Stage 2 patients (31.3%), p=0.767.
Association between BP and SUA levels
We restricted our evaluation of the relationship between BP and SUA to
69 patients who were not on an ACEi for the reasons describe previously
in Methods. In these patients, the correlation between systolic BP and SUA
was r=-.346 (p=0.010), between systolic BP and HU, r=.278 (p=0.040).
The correlation between diastolic BP and SUA was r=.072 (p=0.600),
between diastolic BP and HU, r=.154 (p=0.262).
Relationship between UP/C and SUA levels
Positive correlations were observed in the combined populations
between UP/C and SUA, r=.188, p=0.036; and between UP/C and HU,
r=.201, p=0.024.
Impact of medications on SUA levels
The SUA was 5.77 ± 1.64 mg/dl in the 56 patients who were receiving
an ACEi versus 5.19 ± 1.47 in the other 69 patients, r=.186, p=.037, n=125.
However, there was no correlation between patients with HU and those
on an ACEi, r=0.058, p=0.518. HU was seen in 4/7 patients on fish oil;
0/6 patients on corticosteroids; 0/5 patients on thiazides, and 3/5 on
angiotensin receptor blockers.
Discussion
The observations made in this study demonstrate clearly that both
HU and obesity/overweight are more prevalent in USA/CAN patients
than similarly aged CZE/GER patients with IgAN, and that HU and
obesity/overweight are associated with one another. The HU findings
are not surprising since epidemiologic studies have documented that
the prevalence of HU in the US population has increased in recent years.
Zhu et al. [16] found that the prevalence of HU in adults increased by
approximately 15% from 1988/1994 to 2007/2008 [14]. Many studies
have shown that HU is a risk factor for progressive disease in adult IgAN
patients (8-10), including those with well-preserved renal function. For
example, Syrjänen et al. [8] and Ohno et al. [9] both found HU in 23% of
IgAN patients with well-preserved GFRs.
Although studies of the impact of HU in children with CKD have been
limited (2), many studies have shown that higher SUA levels in children
are associated with hypertension [3,5,6]. Feig et al. [17] reported that
reducing SUA levels in such patients had a significant beneficial effect
on blood pressure (BP). In addition, Soletsky and Feig [18] reported that
reducing the SUA levels using allopurinol in a group of children with SUA
≥ 5 mg/dl and pre-hypertension, reduced the systolic and diastolic BP
significantly and Assadi [19] found that the combination of allopurinol
and enalapril had a greater effect of BP than enalapril alone. This may
be important in patients with IgAN since hypertension is known to be
an important risk factor and treatment with enalapril or one of the other
angiotensin-converting enzyme inhibitors has become standard therapy.
The importance of obesity and overweight in adult patients with IgAN
was first highlighted by Bonnet et al. in 2001 [12]. Bonnet et al. found that
being overweight at the time of diagnosis of IgAN correlated significantly
with more severe renal biopsy lesions and increased levels of proteinuria
and favored the subsequent development of both hypertension and
deterioration of renal function. This issue was “re-visited” by the same
group 12 years later in a larger number of patients (333 versus 162 in the
original report), at which time the prevalence of obesity (10.3% versus
9.3%) and overweight (30.8% versus 32.1%) were essentially unchanged
[13]. At the latest follow-up, there was a greater prevalence of CKD Stage
3 or more of the overweight/obese group (43.3% versus 21.0%, p<0.0001)
and more of them progressed to dialysis or death.
The role of obesity in determining the severity of IgA nephropathy was
studied in Japanese patients by Tanaka et al using quantitative analysis
of renal biopsy features in 2009 [20]. They found that obese patients had
significantly larger glomeruli (p<0.0001), thicker glomerular basement
membranes (p<0.001), and more marked proteinuria than the non-obese
group (p<0.05).
Kovács et al. addressed the prevalence of obesity, as well as other
components of the metabolic syndrome, in 223 Hungarian patients with
IgAN [21]. Sixty-eight (31%) of the patients were obese (BMI ≥ 30 kg/
m2
). Patients with obesity had a greater risk of progressing to CKD Stage
3. It is noteworthy that HU was also found to be an independent risk
factor for progression, but this was not mentioned in the other papers that
examined the role of obesity. The harmful impact of the combination of
HU and obesity deserves additional study in the future.
Our observations regarding the high prevalence of HU and obesity/
overweight in children and adolescents with IgAN in the USA/CAN raise a
number of questions with respect to causation, risk and treatment options.
We did not collect diet histories in our patients but a higher consumption
of certain foods containing fructose is a factor that has been proposed
for the increasing prevalence of hypertension, HU, cardiac disease, and
obesity in the USA population in general; it may also be playing a role
in the USA/CAN pediatric population with IgAN [22]. Although, it is
not possible at the present time to determine the optimal approach to
the management of HU and obesity in children with IgAN, appropriate
dietary recommendations should certainly be adopted. Hopefully, the
issue will be addressed in future clinical trials. In the meantime, it will be
up to physicians caring for the patients with IgAN+HU and/or obesity to
discuss the pros and cons of current options with the parents and child
and determine the therapy on an individual basis.
Acknowledgments
The authors wish to express our thanks to all of the investigators
and coordinators who assisted with patient identification in Canada,
Germany, the Czech Republic and the USA who recruited and studied
the patients. The participating investigators in the USA and Canada are
listed in references [23] and [24]. We also thank Mrs. Gena Garcia and
Mrs. Gina Du Par at Baylor Scott & White Health for their assistance in
the production and submission of this manuscript.
Support and Financial Disclosure
The clinical trials from which the data were derived for the USA and
Canadian patients were funded by:
a) National Institute of Diabetes and Digestive and Kidney Diseases
grant ROI DK 49368.
b) Roche Laboratories Inc.
The study of the Czech and German patients was supported by MH
CZ – DRO, University Hospital Motol, Prague, Czech Republic 00064203.



