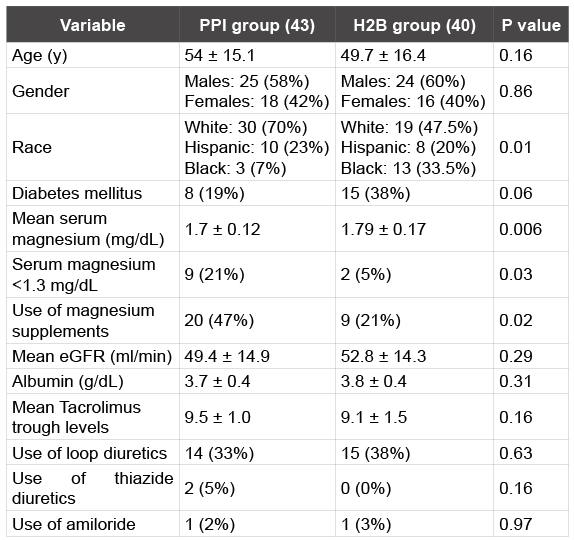
Table 1: Baseline characteristics of study population

Mohamad N Alhosaini1,2 David J Leehey1,2 Kavitha Vellanki1,2,*
1Department of Medicine, Loyola University Medical Center, Maywood, IL, USA*Corresponding author: Kavitha Vellanki, Department of Medicine, Loyola University Medical Center, Maywood, IL, USA, Tel: 708 216 4450; Fax: 708 216 4060; E-mail: kpotluri@lumc.edu
Hypomagnesemia is common in the early post-transplant period and is often associated with CNI use. PPIs are preferred choice of agents for stress ulcer prophylaxis and have been associated with hypomagnesemia especially when coupled with diuretic use. We postulated that PPI use in the post-transplant setting increases the severity of hypomagnesemia associated with CNI use compared to H2B. A total of 83 patients were included in the study, 43 were on PPI and 40 on H2B. There was a statistically significant difference between the two groups with mean magnesium levels being 1.70 ± 0.12 mg/dL in PPI group vs. 1.79 ± 0.17 mg/dL in H2B group (P = 0.006). Severe hypomagnesemia (defined as serum magnesium of <1.3 mg/dl) was more common in the PPI group than H2B (9/43 [21%] vs. 2/40 [5%], P = 0.03) despite oral magnesium supplementation being higher in PPI group (47% vs. 21%, P = 0.02). In conclusion, severe hypomagnesemia and oral magnesium supplementation are associated with the use of PPIs in renal transplant patients.
Hypomagnesemia; Kidney transplantation; Proton pump inhibitors; H2 blockers
CNI: Calcineurin Inhibitors; CsA Nephropathy: Calcineurin induced Nephropathy; eGFR: estimated Glomerular Filtration Rate; H2B: Histamine 2 receptor Blockers; MDRD: Modified Diet in Renal Disease; NODAT: New Onset Diabetis after Transplantation; PPI: Proton Pump Inhibitors
Hypomagnesemia is a frequent complication in renal transplant recipients, particularly in the early post-transplant period, which is mainly attributed to calcineurin inhibitors (CNI) as well as to the use of diuretics [1-3]. Animal studies have shown down regulation of magnesium (Mg2+) transport proteins in distal convoluted tubules as a potential pathogenic mechanism for CNI induced urinary magnesium loss [4]. On the other hand, hypomagnesemia has been associated to the long term use of proton pump inhibitors (PPIs) in different patient populations, especially when coupled with diuretic induced urinary magnesium excretion [5]. PPIs inhibit intestinal absorption of magnesium, thus impairing the normal compensatory mechanism in the face of urinary magnesium loss [6]. Stress ulcer prophylaxis in the immediate post-transplant period is a common clinical practice with many centers using proton pump inhibitors (PPIs) as the preferred drug of choice over histamine-2 receptor blockers (H2Bs). Hence, it seems reasonable to postulate that PPI use in post-transplant setting might increase the severity of hypomagnesemia associated with CNI use relative to patients taking CNI and H2B. To address this question, we reviewed the prevalence and severity of hypomagnesemia in post kidney transplant population at our center and compared patients who received PPI vs. H2B in the post-transplant period.
Data was gathered by retrospective review of charts of post kidney transplant patients who had multiple regular follow-up visits and labs done as per standardized protocols. Only laboratory values obtained in the outpatient setting were collected for the study. The follow up period was one year. The study was approved by the IRB.
The drug of choice for routine stress ulcer prophylaxis post kidney transplant was switched from ranitidine (H2B) to pantoprazole (PPI) in April 2011 due to change in protocol based on physician preferences and hence, all adult patients who received a kidney transplant at our center between January 2010 and December 2012 were included in the study. All patients who received a kidney transplant after April 2011 were automatically placed on pantoprazole 40 mg daily for stress ulcer prophylaxis unless any specific issues. Patients transplanted prior to April 2011 were routinely placed on ranitidine 150 mg twice daily for stress ulcer prophylaxis. No patient was switched from H2B to PPI retroactively due to protocol change. Data were collected retrospectively up to one year post-transplantation. Exclusion criteria included age less than 18, patients who received simultaneous liverkidney or kidney-pancreas transplantation, unknown duration of use of H2B or PPI, missing data, delayed or failed allograft function and death within the first year of transplantation.
Induction therapy consisted of either basiliximab or thymoglobulin and corticosteroids with all patients receiving a dose of 500 mg IV of methylprednisolone preoperatively. Calcineurin inhibitors (Tacrolimus or Cyclosporine), mycophenolic acid (myfortic) and prednisone were used for maintenance immunosuppression. All patients received Pneumocystis jiroveci and Cytomegalovirus (CMV) prophylaxis with trimethoprimsulfomethoxazole or pentamidine for one year and valganciclovir for 3-6 months (based on donor-recipient CMV status) respectively posttransplantation.
One year follow-up data were collected on all patients included in the study and data included age, gender, race, date of transplant, mean serum albumin, magnesium and creatinine levels (average of all outpatient readings within a year post-transplant), use of magnesium supplements, history of gastrointestinal bleeding or peptic ulcer disease, history of diabetes, use of diuretics, use of anticoagulants (aspirin, clopidogrel or warfarin), maintenance immunosuppressive regimen and mean tacrolimus and cyclosporine levels. Outpatient labs were drawn as per institutional protocol, twice weekly for the first two weeks followed by weekly for the next 4 weeks, every two weeks for up to 3 months and monthly for the first year post-transplant. Patients were divided into two groups based on type of stress ulcer prophylaxis: PPI and H2B groups. The primary outcome was to study the prevalence and severity of hypomagnesemia in each group during the first year post transplantation. Hypomagnesemia was defined as mean serum magnesium of less than 1.8 mg/dL. Severe hypomagnesemia was defined as serum magnesium of less than 1.3 mg/dL at any time within a year post-transplantation. The secondary outcomes included incidence of gastrointestinal bleeding and peptic ulcer disease during the study period.
Student’s t-test was used to compare the association between continuous variables and Chi-square for categorical variables. Descriptive statistics were expressed as mean + standard deviation (SD). A p-value < 0.05 was considered statistically significant. Stata 12.0 statistical software package was used for data analysis.
A total of 187 patients received kidney transplants at our center during the study period (January 2010 to December 2012). Patients who were taking H2B or PPI for the whole year, as per medication review, were only included in the final analysis. A total of 83 patients were included in the final analysis after excluding 104 patients (75 who were not taking H2B or PPI at one year post-transplant or had missing data, 13 with delayed graft function, 9 died within a year, 6 received combined liver kidney transplant and one was <18 years). All patients were on CNI (all but 5 patients were taking tacrolimus with the other 5 receiving cyclosporine), mycophenolic acid and prednisone for maintenance immunosuppression. Of the 83 included in the study, 43 patients were on PPI at one year post transplantation and 40 on H2B. Baseline characteristics of the two groups are summarized in (Table 1). Mean age and eGFR (estimated glomerular filtration rate using MDRD formula) was similar in the two groups. There were relatively more Caucasians on PPI compared to African-Americans and there were more diabetic patients taking H2B. Of note, significantly more patients in the PPI group were on oral magnesium supplementation at one year post kidney transplantation compared to the H2B group (47% vs. 21%, P = 0.02). Oral magnesium supplementation varied between 400-1600 mg/day in divided doses in both groups and only one patient in PPI group received intravenous magnesium infusion for severe hypomagnesemia. 67% of the study population (57 out of 83) had hypomagnesemia (mean serum magnesium < 1.8 mg/dl) at one year posttransplantation. There was a statistically significant difference between the two groups with mean magnesium levels being 1.70 ± 0.12 mg/dL in PPI group vs. 1.79 ± 0.17 mg/dL in H2B group (P = 0.006) (Table 1). The prevalence of hypomagnesemia (mean serum magnesium of < 1.8 gm/ dl) was higher in the PPI group compared to H2B group (33/43 vs. 24/40 respectively, (Figure 1) but this did not reach statistical significance, higher prevalence of oral magnesium supplementation in the PPI group being a major confounder. However, severe hypomagnesemia (defined as serum magnesium of < 1.3 mg/dl) was more common in the PPI group than H2B (9/43 [21%] vs. 2/40 [5%], P = 0.03, (Figure 2) despite oral magnesium supplementation being higher in PPI group. None of the patients in either group had GI bleeding complications during the study period.
Over the last decade, PPI use has significantly increased in the US [7] and largely replaced H2B as the agent of choice for stress ulcer prophylaxis in the post- transplant setting. Higher efficacy, ease of administration and safety profile are some of the reasons cited for the growing popularity of PPIs. Despite evidence from a randomized control trial showing that omeprazole (PPI) is not superior to ranitidine (H2B) in preventing GI bleeding in the post-transplant period [8], PPIs are considered to be more efficacious. However, in recent years, the safety profile of PPIs has come into question with observational studies suggesting an excess mortality risk with PPI use in the elderly population [9]. There is concern that alteration in gut flora from PPI may lead to an increased risk of infection (Clostridium difficile colitis and community acquired pneumonia) [10,11], increased risk of fractures [12], and interference of absorption of major nutrients/medications. Moreover, PPIs have been associated with hypomagnesemia from decreased magnesium absorption from the gut, which could increase mortality risk.

Table 1: Baseline characteristics of study population
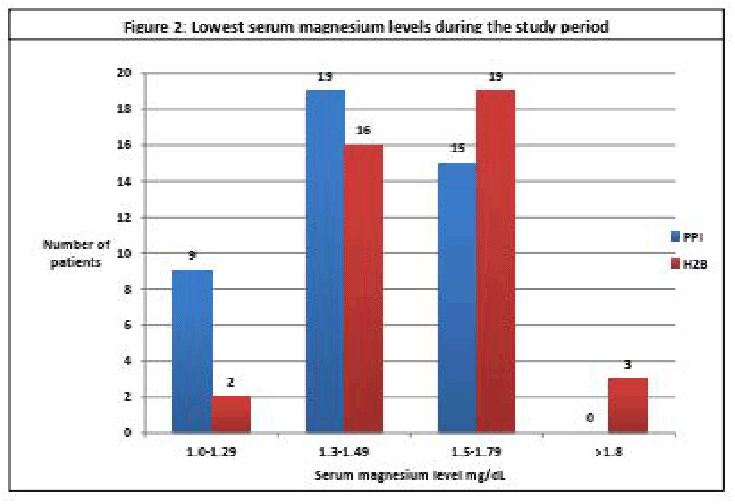
Figure 1: Mean serum magnesium levels within a year post-transplant
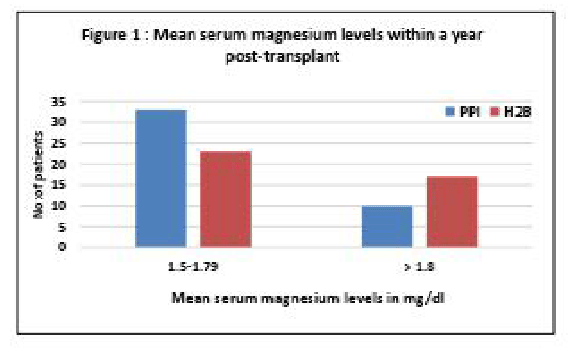
Figure 2: Lowest serum magnesium levels during the study period
Hypomagnesemia is common after transplantation, develops within a few months, and has been reported to improve over time [1,3]. CNIs have known to cause hypomagnesemia with animal studies showing renal magnesium wasting from down regulation of Mg transport proteins in distal convoluted tubules [4]. PPIs decrease active transportation of Mg in the duodenum and cause hypomagnesemia in patients who have decreased oral intake or urinary Mg wasting [5]. Our findings suggest that the combination of PPI and CNI is associated with more severe hypomagnesemia compared to the combination of H2B and CNI. Moreover, almost half of the patients in the PPI group were on oral Mg supplements compared to only one-fifth of the patients in H2B group. It is likely that increased oral Mg intake in the PPI group masked an even greater difference in prevalence and severity of hypomagnesemia in these patients. In fact, episodes of severe hypomagnesemia (defined by <1.3 mg/dL) were significantly more common in the PPI group despite oral magnesium supplementation. Our study contradicts a recent study that reported serum magnesium levels were not influenced by PPI use in kidney transplant recipients [13]. This study looked into clinical characteristics associated with low serum magnesium levels in kidney transplant recipients and use of PPI (which was in 20% of the study population) was not one of them. There are several methodological differences in how the study was conducted. First, this was a cross-section study that included subjects that were enrolled into another large published cohort. It is unclear if mean serum magnesium level or one lab reading of serum magnesium was considered for the analysis. Second, the duration of PPI use was unknown. Third, the lab cut off values for serum magnesium was different from the two different hospital sites that the patients were enrolled. Fourth, data on magnesium supplementation as well as mean cyclosporine or prograf levels were not available. Nor were the data on severity of hypomagnesemia. And finally, there was no comparison group. Hypomagnesemia in the post renal transplant setting has been associated with poor allograft outcomes and new onset diabetes after transplantation (NODAT). Both animal and human studies have reported an increased rate of decline in allograft function and increased graft loss in chronic cyclosporine induced nephropathy (CsA nephropathy) [14,15]. In a retrospective study of 60 patients with biopsy proven cyclosporine mediated nephropathy, the decline in graft function (measured as creatinine clearance) was more rapid in patients with hypomagnesemia than in patients with normal serum magnesium levels (_8.9 vs. 1 ml/min/ year; P = 0.02). The decline in hypomagnesemic patients was faster despite the creatinine clearance being higher in this group at the time of biopsy (44.3 ml/min vs 37.8 ml/min, P<0.05) [14]. Whether hypomagnesemia per se contributes to CsA nephropathy is up for debate but animal studies have shown inhibition of interstial fibrosis and tubular atrophy with magnesium supplementation in CsA nephropathy. Since tacrolimus and cyclosporine affect the kidney in a similar fashion, the same can be expected with tacrolimus induced nephropathy. New onset diabetes after transplantation (NODAT) is a frequent complication and has an impact on patient and graft survival. Hypomagnesemia is thought to be one of the possible mechanisms responsible for the increased incidence of NODAT in calcineurin based drug regimens apart from direct islet cell toxicity and inhibition of insulin gene expression [16]. Studies have shown increased incidence of NODAT in both kidney and liver transplant patients with hypomagnesemia [16,17]. While we are indeed of large controlled studies to further address these issues, it seems prudent to avoid hypomagnesemia in the post-transplant setting. This observational study does have limitations. It included a relatively small number of patients. There were differences in baseline characteristics between the groups, namely a larger number of African-American patients as well as a somewhat greater prevalence of diabetes in the H2B group. It is possible that socioeconomic status and additional financial burden associated with PPI use played a role in the racial differences noted in our study. Glycosuria is well known to increase urinary magnesium excretion and there is an inverse relationship between glycemic control and serum magnesium levels [18]. In our study, the prevalence and severity of hypomagnesemia was lower in the H2B group despite the greater prevalence of diabetes at baseline. In conclusion, our study shows a possible association of hypomagnesemia to the long term use of PPIs in renal transplantation. While the clinical significance needs to be further elucidated, we believe it is prudent to avoid this preventable complication. Taking into account that the study of Van Ende et al. [13] recently suggested that PPI use does not influence serum magnesium levels in kidney transplant recipients, well designed; prospective cohort studies may further clarify this issue. Monitoring of additional parameters like serum calcium, glucose, fractional excretion of magnesium is needed.
Conflict of Interest Declaration: None of the authors have conflict of interests pertaining to the above clinical study.
Download Provisional PDF Here
Article Type: Research Article
Citation: Alhosaini MN, Leehey DJ, Vellanki K (2015) Use of Proton Pump Inhibitors is Associated with Severe Hypomagnesemia in Kidney Transplant Recipients. Int J Nephrol Kidney Failure 2(1): http:// dx.doi.org/10.16966/2380-5498.122
Copyright: © 2015 Alhosaini MN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access