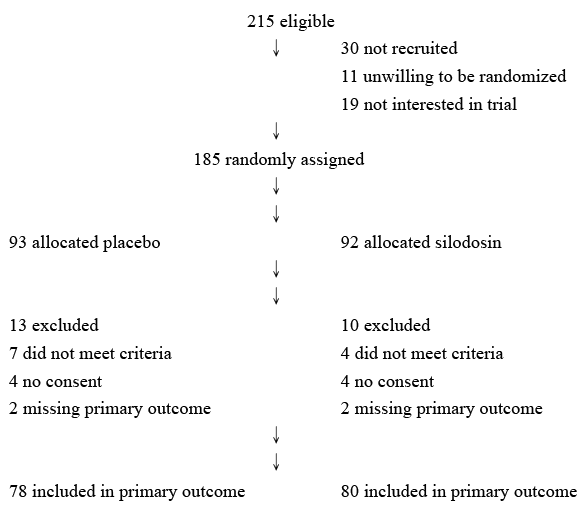
Figure 1: Summary of study disposition
The number of participants declining further follow-up or not responding
is cumulative in the direction of participant flow.

Po-Chao Tsai1 Chung-Jing Wang1* Chien-Hsing Chang1 Hung-Wen Chen2 Chi-Sen Hsu3
1Division of Urology, Department of Surgery, Saint Martin De Porres Hospital, Chiayi, Taiwan*Corresponding author: Chung-Jing Wang, Division of Urology, Department of Surgery, Saint Martin De Porres Hospital, Chiayi, Taiwan, Tel: +886 5 2756000 ext: 1013; Fax: +886-5-2788535; E-mail: jing@stm.org.tw
To evaluate the effect of silodosin on improving symptoms and quality of life in patients with indwelling double-J ureteral stents.
This prospective study lasted from March 2010 to December 2012. All the patients with symptomatic distal ureteral stones with less than a 15-mm diameter were enrolled in this prospective study and were prospectively randomized into two groups. In total, 158 patients underwent the insertion of a double-J ureteral stent after ureteroscopic stone removal. In Group 1, 78 patients were enrolled and they received a placebo for two weeks. Group 2 included 80 patients who received 8 mg of silodosin once daily for two weeks. All patients completed a 10 cm linear visual analogue scale (VAS) to evaluate pain and voiding flank pain, as well as the irritative domain of the International Prostate Symptom Scale (IPSS) before double-J stent removal two weeks later.
The mean VAS for pain was 3.96 ± 1.30 in Group 1 and 1.51 ± 0.75 in Group 2. For voiding flank pain, it was 3.27 ± 1.07 in Group 1 and 1.93 ± 0.87 in Group 2. The mean score of frequency on the IPSS was 3.68 ± 1.01 in Group 1 and 1.54 ± 0.73 in Group 2. The mean score of urgency on the IPSS was 3.83 ± 0.93 in Group 1 and 1.43 ± 0.69 in Group 2. The mean score of nocturia on the IPSS was 2.04 ± 1.00 in Group 1 and 0.66 ± 0.48 in Group 2. The mean score of quality of life on the IPSS was 4.19 ± 0.88 in Group 1 and 1.63 ± 0.74 in Group 2. The mean dosage of buprenorphine was 0.07 ± 0.13 mg in Group 1 and 0.01 ± 0.04 mg in Group 2. The Mann–Whitney U test revealed all p-values were less than 0.001 with significant statistical differences.
Silodosin improved a subset of stent-related urinary symptoms including pain, voiding flank pain, and quality of life.
Silodosin; Double-J stent; Alpha-1A-blocker
IPSS: International Prostate Symptom Scale; LUTS: Lower Urinary Tract Symptoms; BPH: Benign Prostatic Hyperplasia; VAS: Visual Analogue Scale; USSQ: Ureteral Stent Symptom Questionnaire
The placement of a ureteral stent is a common urological intervention. It has been more than three decades since the first description of a cystoscopically placed temporary ureteral stent by Zimskind PD et al. [1], and indications and use have continued to expand [2]. However, the side effects and patient morbidity associated with ureteral stents have been identified as potential health problems [3]. The great variety of complications range from the commonly experienced stent syndrome to the medicolegal dilemma of the forgotten stent [4].
Many researchers have presented validated questionnaires for the assessment of stent-related symptoms and the evaluation of their impact on a patient’s daily life [2]. However, they are complicated and difficult to utilize worldwide, like the IPSS. In addition, most efforts have been aimed toward improving stent materials and design; they have not been of practical clinical use because the alleviation of symptoms has been minimal [5,6].
Silodosin is a highly selective α1-adrenoreceptor antagonist used in the symptomatic treatment of lower urinary tract symptoms (LUTS), especially in male patients suffering from benign prostatic hyperplasia (BPH) [7]. Silodosin is known to inhibit α1-adrenoreceptors that are densely populated in the smooth muscle cells of the lower urinary tract, and therefore, it relaxes them [8,9]. The symptomatic relief of LUTS due to α1-blocker medication is attributed to this relaxation of the lower urinary tract smooth muscle, and several studies have shown reduced stent-related symptoms in patients with ureteral stent placement who received α1-blockers, such as alfuzosin and tamsulosin [10-13]. In the light of these reports, silodosin was used in this study, as it is known to have high uroselectivity to α1-adrenoreceptors for patients with a double-J catheter placement. The effect of silodosin in improving symptoms and quality of life in patients with indwelling double-J ureteral stents was evaluated.
Approval was received from the Institutional Review Board at St. Martin De Porres Hospital in Chia-Yi city, where the work was undertaken. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments, or comparable ethical standards. All patients signed an informed consent form before participating. To detect a 30% difference in the proportion of stent-related complications in the treatment groups, at a significance level of 0.05 and a power of 90%, a sample size of 75 patients per group was calculated. Therefore, 158 patients (125 male and 33 female) in total who underwent the routine insertion of a double-J ureteral stent after total ureteroscopic stone removal were prospectively randomized (random numbers table) into two groups (Figure 1). From March 2010 to December 2012, all patients with symptomatic distal ureteral stones with less than a 15-mm diameter and stone-related hydronephrosis were enrolled in this prospective study. In Group 1, 78 patients (60 males and 18 females; mean age, 50.64 ± 10.58 years old) were enrolled, and they received a placebo for two weeks. Group 2 included 80 patients (65 males and 15 females; mean age, 50.4 ± 9.41 years old) who received 8 mg of silodosin once daily for two weeks. All patients completed the 10-cm linear visual analogue scale (VAS) for evaluating pain and voiding flank pain, as well as the irritative domain of the International Prostate Symptom Scale (IPSS) before double-J stent removal two weeks later. A statistical analysis was performed using the chi-square test and the Mann–Whitney U test, as appropriate.
The exclusion criteria included benign prostatic hyperplasia-related lower urinary tract symptoms (IPSS greater than 7); a history of interstitial cystitis, chronic cystitis, chronic prostatitis, or stent insertion; and chronic medication with alpha-blockers or analgesics. During the stenting period, all patients were prescribed pipemidic acid trihydrate at 250 mg twice per day to minimize urinary tract infections, and they were allowed the use of 2 mg of sublingual buprenorphine on demand. The overall dosage was documented and compared. The same silicone-coated double-J ureteral stent design (Cliny, Japan) was inserted in all patients. The stent size was fixed (Fr 7) and the length was adjusted by body height and applied to all patients after ureteroscopic stone removal under intravenous general anesthesia. The stent’s correct position was confirmed with a plain kidney-ureter-bladder x-ray. The double-J stents were removed with a cystoscope. All consenting patients were fully informed regarding the potential side effects of silodosin; however, they were unaware of whether they were receiving a placebo or silodosin. In addition, the physician who administered the medication was unaware of the treatment group of the patient.

Figure 1: Summary of study disposition
The number of participants declining further follow-up or not responding
is cumulative in the direction of participant flow.
In total, 158 patients completed the study. No significant statistical difference was observed among patient age, gender distribution, body height, stone size, or operative time (Table 1). No complications occurred after double-J stent placement.
As shown in table 2, the mean VAS for pain was 3.96 ± 1.30 in Group 1 and 1.51 ± 0.75 in Group 2, and for voiding flank pain, it was 3.27 ± 1.07 in Group 1 and 1.93 ± 0.87 in Group 2. The mean score of frequency on the IPSS was 3.68 ± 1.01 in Group 1 and 1.54 ± 0.73 in Group 2. The mean score of urgency on the IPSS was 3.83 ± 0.93 in Group 1 and 1.43 ± 0.69 in Group 2. The mean score of nocturia on the IPSS was 2.04 ± 1.00 in Group 1 and 0.66 ± 0.48 in Group 2. The mean score of quality of life on the IPSS was 4.19 ± 0.88 in Group 1 and 1.63 ± 0.74 in Group 2. The mean dosage of buprenorphine was 0.07 ± 0.13 mg in Group 1 and 0.01 ± 0.04 mg in Group 2. The Mann–Whitney U test revealed all p-values were less than 0.001 with significant statistical differences. Only four patients in the silodosin group experienced adverse effects associated with the medical therapy (transient hypotension, asthenia, syncope, palpitations, and retrograde ejaculation), whereas no patients who suspended medical therapy experienced the adverse effects. Only four patients in Group 2 needed sublingual buprenorphine therapy, whereas 20 patients in Group 1 required this regimen, and 11 patients suffered from adverse effects (dizziness, anorexia, and vomiting). A statistically significant difference was noted (p=0.0002).
Indwelling double-J ureteral stents have become routine in the management of a variety of urinary tract diseases. Stents prevent urinary tract obstruction, divert urine, allow for faster tissue healing, dilate the ureter, and assist in stone passage [2]. However, the ideal stent is not yet available [3,4,14]. Many patients will experience significant stentrelated morbidity, and an additional procedure to remove the stent is usually needed [3,15] To minimize the above-mentioned problems, [16,17] new double-J stents with tapered distal ends made from a hydrophilic material have been developed, and stents created from new biodegradable or tissue-engineered materials may eliminate the need for stent removal in the future [18].
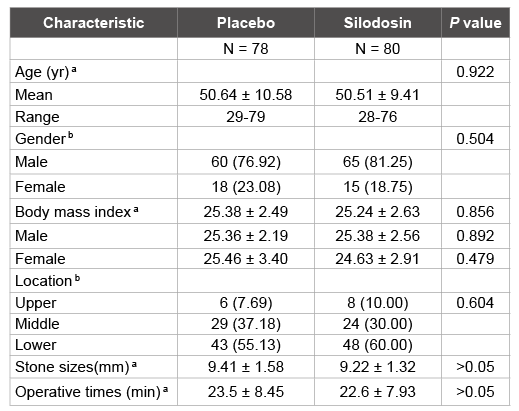
Table 1: Patients characteristics
Values are presented as mean ± standard deviation or number (%).
a
Mann–Whitney U test
b
Chi-square test

Table 2: Randomization study results
Values are presented as mean ± standard deviation.
***p < 0.001
a
Mann–Whitney U test
Medical therapies, especially the use of α1-blockers, are also used to overcome stent-related symptoms. The α1-blockers were introduced for the treatment of male LUTS in the early 1990s, and they have become the most widely used drugs to treat LUTS [19]. The α1-blockers act as an antagonist to α1-adrenoreceptors in the smooth muscle cells of the lower ureter, bladder, and prostatic urethra. The inhibition of α1-adrenoreceptors relaxes the smooth muscles in such regions and thereby prevents spasm and reduces bladder outlet resistance [8,9]. These effects contribute to reducing the intravesical pressure during micturition and can thereby decrease the renal reflux of urine that causes the flank pain in patients with ureteral stents [10]. Previously, a randomized, prospective study of 55 patients with indwelling ureteral stents receiving 10 mg of alfuzosin daily was conducted and revealed a significant decrease in urinary symptoms and flank pain [10]. The patients in this study were asked to complete the ureteral stent symptom questionnaire (USSQ) developed by Joshi et al. [20] to evaluate stent-related symptoms.
Another study, reported in 2006, showed an alleviation of stent-related urinary symptoms and pain in patients receiving 10 mg of alfuzosin daily for four weeks, and the results were also evaluated by applying the USSQ [11]. Further, the patients in the alfuzosin group were found to have a significantly better preservation of sexual function and general health [11]. In this study, 8 mg of silodosin was applied daily for two weeks in patients with ureteral stents, and the stent-related symptoms were evaluated using the irritative domain of the IPSS and the 10-cm linear VAS.
Silodosin is a highly selective antagonist for the α1A-adrenergic receptor subtype [7]. It was first introduced under its original name of KMD-3213 in 1995, and it is used for the treatment of LUTS in BPH patients [21]. Several studies have shown the high selectivity of silodosin toward the α1A-adrenergic receptor subtype. A study conducted in 2006 reported that the affinity for the α1A-adrenergic receptor subtype in silodosin was 162 times and 55 times stronger than that of the α1B- and α1D-adrenergic receptor subtypes, respectively [14,22,23]. Due to its high selectivity for the α1A-adrenergic receptor subtype, which is predominant in the smooth muscles of the lower urinary tract, silodosin causes much fewer adverse cardiovascular effects, such as orthostatic hypotension, compared to other α-blockers when used for the treatment of LUTS [7]. This implies that silodosin may be a safer drug than other α1-blockers, especially in patients suffering from cardiovascular disease. None of the patients in this study who received silodosin experienced any cardiovascular events or other serious adverse effects during the study period.
In this study, silodosin significantly reduced the prevalence of lower urinary tract symptoms. Stent-related pain was significantly lessened in patients receiving silodosin, who required significantly fewer analgesics. Silodosin reduced not only pain during voiding, but also loin pain, possibly by reducing urine reflux from improved bladder neck relaxation.
The potential limitations of this study are acknowledged, as reported by Deliveliotis C et al. [11] in that only a single stent, design, and material were evaluated; however, it has been demonstrated that the degree of stent-related symptoms is not associated with the stent characteristics (composition, style, length), placement techniques, or body height or gender [24-26]. Besides, the utilization of a single stent size in a study can minimize trial variability. Ureteroscopic sto*4/ne manipulation was performed with the routine insertion of a ureteral stent for four weeks until the participants completed the IPSS and terminated treatment (silodosin or placebo). However, it has been shown that stent-related symptoms and general patient tolerance remained unchanged with time [27]. The primary objective of this study was to evaluate whether the concept of using an α-1A-blocker is justified. Future randomized prospective studies of a larger sample of consecutive patients with a longer follow-up might potentially overcome these limitations and compare the morbidity of stents with different characteristics and insertion indications.
Another limitation was that no comparison was made with the use of other α1-blockers, such as alfuzosin and tamsulosin, a comparison that would have more precisely defined the exact effect of silodosin in stentrelated symptoms. Finally, the trend of many similar recent studies has been to use the USSQ, rather than the IPSS and VAS. Applying the USSQ in this study may have been a better choice both in the evaluation of stentrelated symptoms and in comparing the results with other studies.
The double-J stent has become an integral part of urologic intervention; however, stent-related morbidity is a reality in the great majority of patients. The administration of a selective α1-blocker, such as silodosin, improves stent-related urinary symptoms, pain, and voiding flank pain. Future research is needed to refine the exact role of selective α1-blockers in managing stent-related symptoms.
All authors declare there are no potential conflicts of interest relevant to this article “Effects of Silodosin on Lower Urinary Tract Symptoms Due to a Double-J Stent: A Prospective Randomized Study.”
Download Provisional PDF Here
Article Type: Research Article
Citation: Tsai PC, Wang CJ, Chang CH, Chen HW, Hsu CS (2015) Effects of Silodosin on Lower Urinary Tract Symptoms Due to a Double-J Stent: A Prospective Randomized Study. Int J Nephrol Kidney Failure 1(3): http://dx.doi.org/10.16966/2380-5498.118
Copyright: © 2015 Tsai PC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access