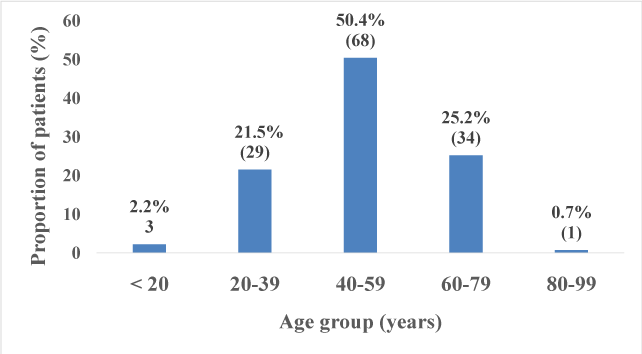

Full Text
Mahamat Abderraman G 1*, Elhadji Fary KA2, Mouhammadou Moustapha Cisse2, Ahmed Tall Lemrabott2, Maria Faye2, Abdou Niang2, Boucar Diouf2,
1 Head of clinic assistant, nephrology-unit, Hôpital de la Renaissance (N’Djamena – Chad), Chad2 Nephrology unit - CHU Aristide le Dantec Dakar-Senegal
*Corresponding author: Mahamat Abderraman G, Nephrologist - Head of clinic assistant, Hôpital de la Renaissance (N’Djamena – Chad), Chad, Tel: 00.235.66.61.95.95/00.235.99.71.81.81: E-mail: zalba2001@yahoo.fr
Abstract
The bone and mineral disorders related to chronic kidney disease (BM-CKD) are common among chronic hemodialysis patients with increased overall mortality due to secondary hyperparathyroidism and vascular calcifications.
Patients and method: This is a retrospective, descriptive and analytical multicenter study made in the hemodialysis unit of the Aristide Le Dantec hospital in Dakar and 2 private hemodialysis units concerned with 135 patients. The objective of the study was to determine the epidemiological, clinical and laboratory phosphocalcic disorders of chronic hemodialysis patients in Dakar. Then these data were compared with the latest targets KDIGO 2009 to make recommendations for good practice against doctors.
Results: The average age was 50 years with a sex ratio of 1.04. Average time in hemodialysis patients was 47 months. The dialysis medium frequency per week was 2.86 per week. The average duration of dialysis was 4.1 hours. All centers used dialysate bath at 1.5 mmol/l calcium.
The main causal nephropathy was represented by the common nephroangiosclerosis (51.1%), nephropathy indeterminate (14.8%) and chronic glomerulonephritis (10.4%).
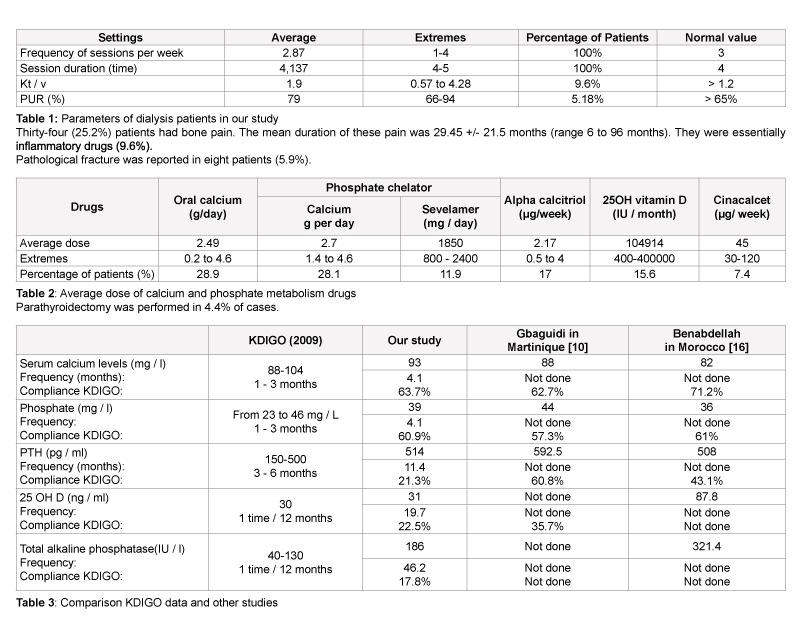
The overall prevalence of calcium phosphate disorders was 23% for hypocalcemia, hyperphosphatemia for 30.8%, 42.6% for hyperparathyroidism, 16.3% for the decline in reserves of native vitamin D and 8.1% for high total alkaline phosphatase (TAP). The average frequency of dosing of these different parameters calcium phosphate was 4.1 months for serum calcium and phosphate, 11.4 months for PTH, 19.7 months for the 25OH Vitamin D and 46.2 months for Total alkaline phosphatase. Regarding the percentage of patients who were in the KDIGO standards, there was 63.7% for serum calcium; 60.9% for phosphate; 44.7% for iPTH; 21.5% for the 25OH Vitamin D and 9.6% for total alkaline phosphatase. Phosphate binders were prescribed in 28.1% of cases for the calcium carbonate and in 11.9% of cases for sevelamer. Oral vitamin D was prescribed in 15.6% of cases, calcitriol in 17% of cases and cinacalcet in 7.4% of cases. Parathyroidectomy was performed in 4.4% of cases.
Control status phosphocalcic of chronic hemodialysis is a hot topic. Its complications are responsible for a global and cardiovascular morbidity and mortality. Preventing BM-CKD requires good control of hyperphosphatemia and secondary hyperparathyroidism.
Keywords
Bone and mineral disorders; Secondary hyperparathyroidism; Dialysis; Chronic renal failure; Senegal
Abbreviations:
BM-CKD: Bone and Mineral disorders related to Chronic Kidney Disease; KDIGO: Kidney Disease Improving Global Outcomes; CRF: Chronic Renal Failure; CKD: Chronic Kidney Disease; iPTH: intact Para Thyroid Hormone
Introduction
The bone and mineral disorders related to Chronic Kidney Disease (BM-CKD) is a major complication of Chronic Renal Failure (CRF). They are defined according to the recommendations KDIGO 2009 (Kidney Disease Improving Global Outcomes) as disturbances of mineral metabolism and their effects on the skeleton (bone fragility by osteopenia) and soft tissue (joint metastatic calcification, vascular, pulmonary and cardiac. These disorders are characterized by changes in the homeostasis of phosphate and calcium and its regulatory elements, mainly in iPTH and 1, 25 OH vitamin D; profound changes in bone and / or bone remodeling structure and the occurrence of vascular calcification [1]. The BM-CKD is directly correlated to the degree of chronic kidney disease. Prevalence of CRF in Australia, Europe and Japan is between 6 and 16% [2]. In Africa, the CKD is responsible for 4-22% of deaths [3]. In Senegal, the hospital incidence is estimated at 87 new cases per year [4]. Overall mortality in hemodialysis patients with BM-CKD is increased due to secondary hyperparathyroidism [5]. Patients with CKD are at high risk of cardiovascular disease with cardiovascular death rate 10 to 20 times higher in dialysis patients than in the general population [6]. This prevalence is largely due to hyperphosphatemia and its bone consequences and vascular calcifications including coronary and valvular whose risk is five times higher [7]. Phospho-calcium disorders remain a major concern for nephrologists due to their impact on the prognosis of patients with a particularly high morbidity and mortality in hemodialysis patients [8]. Previous studies have shown that serum levels of calcium, phosphorus and parathyroid hormone (iPTH) were only controlled in some hemodialysis patients [8].
Comparable work in our study was conducted in Europe Laradi et al. [9], and Martinique by Gbaguidi et al. [10]. In Africa, there were few studies conducted. However, Benabdellah et al. had made similar work to ours, assessing the phosphate profile of 66 hemodialysis patients in Oujda (Morocco). In Senegal, no study has been done on this subject. That is why we conducted this study to study the profile phosphate chronic hemodialysis, we conducted this study in Senegal. The objective was to determine the phosphate status, the prevalence of secondary hyperparathyroidism, assess the prescribed treatment and compare the latest KDIGO recommendations [11,12].
Patients and Methods
This was a retrospective, descriptive and analytical study including records of all chronic hemodialysis for at least 6 months from 1 January 1999 to 01 January 2014, carried out in three hemodialysis centers of Dakar. The parameters studied were epidemiological, clinical, biological, morphological and therapeutic. These parameters were then compared with the recommendations KDIGO 2009. Categorical variables were represented by gender, occupation, bone pain and pathological fractures. Quantitative variables were represented by age, years on dialysis, the number of hours per dialysis session, the number of dialysis sessions per week, the PUR (percentage of urea reduction), Kt / V, biochemical tests: hemoglobin, CRP, phosphate, calcium, iPTH, 25OH vitamin D, total alkaline phosphatase and albumin. The collected data were entered and analyzed using SPSS 18 software.
Results
The total number of patients included in the study was 135. The average age was 50.24 +/- 14.59 years with extremes of 19 and 93 years. The sex ratio was1.04 (Figures 1-5) and (Tables 1 and 2).
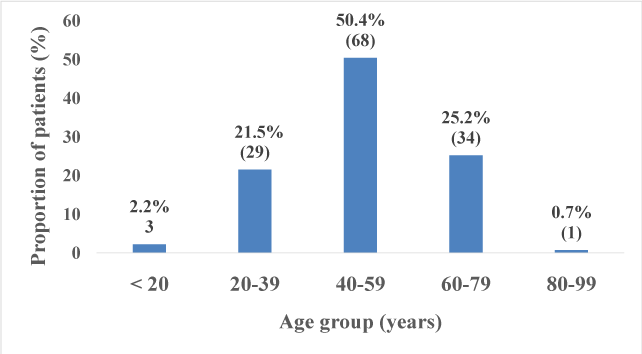
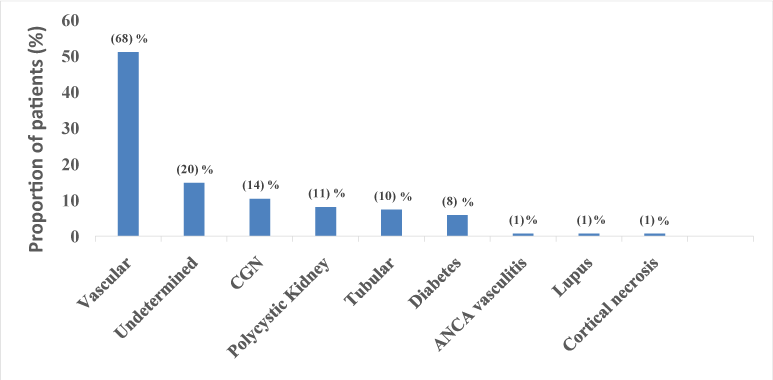
Figure 2:Distribution of initial nephropathy The main occupations were senior managers with 8.9% (n=12), professional occupations with 5.2% (n=7) and students accounted for 3% (n=4). There were 14.8% (n=20) of the unemployed and 68.1% (n=92) of unspecified jobs. The average duration of hemodialysis patients was 47.3 +/- 28 months. Dialysis average frequency per week was 2.86 per week. The average duration of dialysis was 4.1 hours. All centers used dialysate bath to 1.5 mmol/ l of calcium.
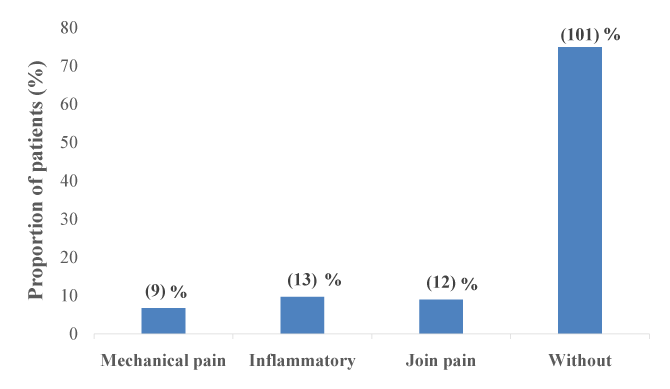
Figure 3: Distribution of patients by bone pain The overall prevalence of calcium phosphate disorders was 23% for hypocalcemia, hyperphosphatemia for 30.8%, 42.6% for hyperparathyroidism, 16.3% for the decline in reserves of native vitamin D and 8.1% for high total alkaline phosphatase. The average frequency of dosing these settings calcium phosphate was 4.1 months for serum calcium and phosphate; 11.4 months for iPTH; 19.7 months for the 25OH Vitamin D and 46.2 months for total alkaline phosphatase.
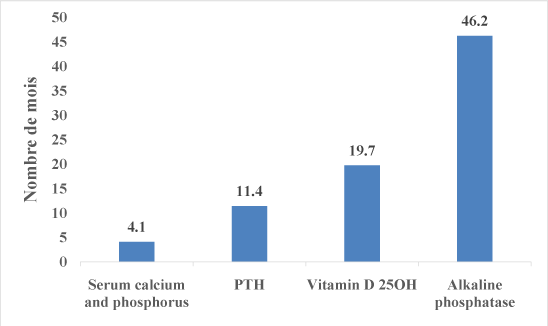
Figure 4:Average frequency for the realization of calcium and phosphate levels Regarding the percentage of patients who were in the KDIGO standards, there was 63.7% for serum calcium; 60.9% for phosphate; 44.7% for iPTH; 21.5% 25OH Vitamin D and 9.6% for total alkaline phosphatase. Morphologically, 14.1% of patients had bone demineralization on plain radiographs of the skeleton. A cardiac ultrasound, 15 patients (11.1%) had vascular calcifications. Among them, 1/3 had a calcification of the aortic cusps, 1/3 and 1/3 mitral calcification of the abdominal aorta. Therapeutically, there were 45 patients or 33.3% who received no medical treatment and 90 patients or 72.7% who were taking at least one treatment. Phosphate binders were prescribed in 28.1% of cases for the calcium carbonate with an average dose of 2.7 g per day and in 11.9% of cases for sevelamer with a mean dose of 1850 mg per day. Oral vitamin D was prescribed in 15.6% of cases, calcitriol in 17% of cases the dose and cinacalcet in 7.4% of cases with average doses were respectively 104 914 IU per liter, 2.17 µg per week and 45 mg per week.
Discussion
Our study revealed that 17.78% of patients had their three main phosphocalcic parameters (serum calcium, phosphate and iPTH) in target values KDIGO. Benabdellah et al. Morocco, Gbaguidi et al. Martinique [10] and Laradi A et al. [9], France also found a prevalence of around 10% of patients reached values targets for 3 phosphocalcic parameters according to the latest KDIGO recommendations. The average age of our patients was 50.24 +/- 14.594 years, approaching that of Morocco with 49.8 years. The study population was young; with the most represented age group is the 40 to 59 years. We observed a statistically significant correlation between age and vascular calcifications. These data are comparable to the study of Goodman WG et al, who had scored the same result [13].
Vascular nephropathy was the most represented (51.1%), followed by the kidney indeterminate causes (14.8%). In Martinique and Morocco, the results were similar, suggesting that vascular nephropathy is the leading cause of dialysis setting. This could be explained by the high prevalence of hypertension (55-85%) [14], poor monitoring of patients and high salt intake in our country. Clinically and mixed inflammatory bone pain were noted in more than a quarter of patients. The probable causes are related to osteopathy and mineral bone disorder, defect making native vitamin D and inflammatory status of patients with 22.2% of patients had an elevated CRP. In multivariate analysis, there was a significant association between bone pain, pathological fractures, and blood levels of vitamin D.
Eight patients (5.9%) had a pathological fracture like the Moroccan study and French. In bivariate analysis, we found a significant association between bone pain, calcium phosphate binders (p=0.026) and pathological fractures (p< 0.000). By against, Meunier et al. [15] in their study on the clinical validation of dietary measures to prevent calcium phosphate disorders did not find any significant association between fracture prevention, secondary hyperparathyroidism and osteomalacia patients with renal impairment.
The mean serum phosphate was 39.3 +/- 12.46 mg/l with extremes of 11 and 85 mg/l with 60.9% of patients who had a serum phosphate in KDIGO standards, consistent with the literature data African [11,12,16]. There were 30.8% of patients who had hyperphosphatemia and 8.3% hypophosphatemia. Hypophosphatemia could be explained by malnutrition (14.1% had hypoalbuminemia) and the inflammatory state (22.2% had elevated CRP) of our patients. Hypophosphatemia is a risk of death at 12 months related to malnutrition according to recent French data (photograph) [12,17]. We did not find any significant correlation in bivariate analysis between hyperphosphatemia and vascular calcification of our patients. But London et al. [18] in a work done in 2003, showed the opposite. The average frequency of realization of serum calcium and phosphate was 4.1 months. It was slightly above the recommended standards (1- 3 months) [11]. An effort to reduce hyperphosphatemia remains. We suggest using poor dialysate calcium and increasing the dose of calcium carbonate (8 to 12 g per day) or increase the prescription of sevelamer [19]. The mean iPTH was 514.8 +/- 409.88 pg/ml with a range of 57 to 2094 pg/ml. 44.7% of patients were within the recommended normal range it was noted that. Our data are well below the other results of world literature, particularly in France [9] with 68.2%; in Martinique [10] with 60.8% and Morocco [13] with 43.1%. However, 42.6% of our patients had hyperparathyroidism. There was no significant correlation in multivariate analysis between PTH and otherphosphocalcic anomalies as demonstrated by Coen et al. [20].
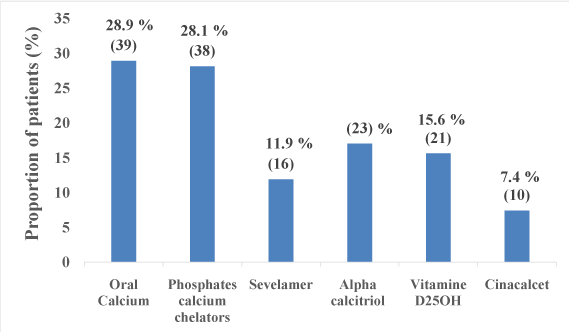
Figure 5: Proportion of patients under medication phosphate metabolism
The average frequency of realization of PTH was 11.4 months. This amounted to almost double the KDIGO targets (3-6 months) [12].
It was noted that 21.5% of patients had normal dosage of vitamin D against 16.35% native that had low reserves. Fortunately, this rate is better compared to that obtained by Laradi in France [9] which was 70% lower reserves. But, it was lower than the result of Elouazzani in Morocco which noted an average rate of 61 ng/l. Our results could be explained by the cost of vitamin D dosage but also by its low prescribing by nephrologists. The average frequency of realization of vitamin D was 19.7 months or more than 1.5 times the recommended standards (once a year) [11,12]. Failure of vitamin D is related to hyperparathyroidism, independently of calcitriol [21], to a defect in bone mineralization, to a greater incidence of epithelial cancers, muscle weakness, infections and anomalies immunity [22]. Vitamin D has three types of effects on the vascular system: inflammatory, reducing myocardial hypertrophy, inhibition of the renin angiotensin system [23]. In addition, patients with vitamin D deficiency have a higher mortality rate [24]. Thus treatment with a form of “inactive” of vitamin D, calcidiol or 25 (OH) D is a new approach for patients with CKD [25]. In multivariate analysis, there was a significant association between bone pain, vitamin D native (p=0.045) and pathological fractures (p= 0.007). Several studies have reported improved survival in hemodialysis patients receiving active vitamin D [26].
Few studies have been conducted to date in the field of phosphate profile for PAL. In our study, 9.6% of patients had levels in the standards recommended by the KDIGO against 8.1% who were at high rates. This could be explained by the high bone turnover of secondary hyperparathyroidism of patients (40.2%). These results were difficult to interpret because only 17.8% of patients have benefited from this review. Its average completion rate was 46.2 months, i.e. more than 3.5 times the recommended duration by KDIGO [11,12].
In our series, 15 patients whether 11.1% had valvular calcifications. These results were comparable to those of Benabdellah et al. Morocco [16] were 6 patients with valvular calcification or 9% of cases. These vascular calcifications could be explained by the average length of time in hemodialysis which was 4 years, and that more than a third of patients had hyperphosphatemia. Lindner et al. noted in their study, morbidity and very high mortality from cardiovascular complications of atherosclerosis [27]. In multivariate analysis a significant correlation between advanced age, years on dialysis and high average dose of ingested calcium carbonate as phosphate binder. But, there was no significant association between vascular calcification and other parameters phosphocalcic, as written by J. Hamburger et al. in their study [28]. By against, several studies have shown that there was a relationship between phosphate and vascular calcification [1] (Table 3).
Conclusion
The BM-CKD are common among chronic hemodialysis patients. Overall mortality in hemodialysis patients with BM-CKD is increased due to secondary hyperparathyroidism. Vascular calcification risk is five times higher, and contributes to increased cardiovascular mortality. Few studies have been conducted in Africa in this regard, particularly in Senegal where hyperparathyroidism represents 42.6% of cases. Prevention and improvement of these disorders requires a compliance with the recommendations KDIGO 2009 on BM-CKD.
References
- Kamel S, Drueke T, Massy Z (2013) Chronic kidney disease - mineral and bone disorders (CKD-MBD. Revue francophone des laboratoires 2013: 29-43.[Ref.]
- Lefigaro (2007) Science and environment. Mise à jour le (21H50). [Ref.]
- Gallé BL (1993) Insuffisance rénale chronique chez le noir africain dans un service de médecine interne pour adultes : étude de 800 cas hospitaliers. Med Abidjan 1460: 260.
- Diouf B, Niang A, Ka EF, Badiane M, Moreira Diop T (2003) Chronic renal failure in one Dakar Hospital Department. Dakar Med 48: 185-188. [Ref.]
- Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, et al. (2004) Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208-2218. [Ref.]
- Massy ZA, Maizel J (2014) Pleiotropic effects of sevelamer: a model of intestinal tract chelating agent. Nephrol Ther 10: 441-450. [Ref.]
- Brunet P (2009) Chronic renal failure: From bone disease to bone and vascular disease. Med Nuc 33: 33-38. [Ref.]
- Pelletier S, Roth H, Bouchet JL, Drueke T, Hannedouche T, et al. (2010) Mineral and bone status in French maintenance hemodialysis patients: A comparison of June 2005 and June 2008. Nephrol Ther 6: 11-20. [Ref.]
- Laradi A, Babinet F, Cremault A (2011) Profil épidémiologique et statut phosphocalcique des patients incidents pris en dialyse entre octobre 2010 et avril 2011. Comparatif avec l’ensemble des patients incidents de l’Observatoire photographe. Nephrol Ther 7: 421. [Ref.]
- Gbaguidi A, Agboton C, Gbaguidi H, Davodoun T, Dueymes JM (2012) Évaluation du profil phosphocalcique des patients hémodialysés. Nephrol Ther 8: 318. [Ref.]
- Cavalier E, Souberbielle JC, Delanaye P (2012) Suivi biologique du métabolisme phosphocalcique chez le patient dialysé : que nous apportent les guidelines du KDIGO en pratique? Immunol Biol Spec 27: 283-285. [Ref.]
- Jean G, Chazot C (2010) The French clinician’s guide to the Kidney disease: Improving global outcomes (KDIGO) for chronic kidney disease-mineral and bone disorders (CKD-MBD). Nephrol Ther 6: 151–157. [Ref.]
- Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, et al. (2006) Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 70: 771-780. [Ref.]
- Simon P (2009) Epidemiology of HTN in dialysis. Nephrol Ther 3: S143-S149.[Ref.]
- Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, et al. (1992) Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med 327: 1637-1642. [Ref.]
- Benabdellah N, El Harraqui R, Karimi I, Khanfri N, Bentata Y, et al. (2012) Troubles du métabolisme minéral et osseux dans une population d’hémodialysés chroniques : évaluation de l’adhésion aux recommandations KDIGO et K/DOQI. Nephrol Ther 8: 318. [Ref.]
- Fouque D, Roth H, London G, Drueke T, Bouchet JL (2009) Shortterm mortality risk factors in a large cohort of hemodialysis patients: importance of nutritional parameters. World Congress of Nephrology, Milano, Italy
- London GM, Guérin AP, Marchais SJ, Métivier F, Pannier B, et al. (2003) Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 18: 1731-1740[Ref.]
- Harbouche L, Shahapuni I, Monge M, Araar B, Rahmouni K, et al. (2006) Place of new treatments for renal osteodystrophy: “non hypercalcemic” 1α OH vitamin D derivatives, non calcic, magnesium, aluminic-phosphatebinder and calcimimetics. Immunol Biol Spec 21: 9-32. [Ref.]
- Coen G, Manni M, Mantella D, Pierantozzi A, Balducci A, et al. (2007) Are PTH serum levels predictive of coronary calcifications in haemodialysis patients? Nephrol Dial Transplant 22: 3262-3267. [Ref.]
- Ghazali A, Fardellone P, Pruna A, Atik A, Achard JM, et al. (1999) Is low plasma 25-(OH)-vitamin D a major risk factor for hyperparathyroidism and Looser’s zones independent of calcitriol? Kidney Int 55: 2169-2177. [Ref.]
- Coen G, Mantella D, Manni M, Balducci A, Nofroni I, et al. (2005) 25-hydroxyvitamin D levels and bone histomorphometry in hemodialysis renal osteodystrophy. Kidney Int 68: 1840-1848. [Ref.]
- Levin A, Li YC (2005) Vitamin D and its analogues: do they protect against cardiovascular disease in patients with kidney disease? Kidney Int 68: 1973-1981. [Ref.]
- Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, et al. (2007) Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int 72: 1004-1013. [Ref.]
- Shoji T, Shinohara K, Kimoto E, Emoto M, Tahara H, et al. (2004) Lower risk for cardiovascular mortality in oral 1alpha-hydroxy vitamin D3 users in a haemodialysis population. Nephrol Dial Transplant 19: 179-184. [Ref.]
- Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, et al. (2003) Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med 349: 446-456. [Ref.]
- Lindner A, Charra B, Sherrard DJ, Scribner BH (1974) Accelerated atherosclerosis in prolonged maintenance hemodialysis. N Engl J Med 290: 697-701. [Ref.]
- Fournier A, Said S, Ghazali A, Sechet A, Oprisin R, et al. (1997) Ostéopathie adynamique de l’urémique : Quelle signification clinique ? : Insuffisance rénale chronique et dialyse = Adynamic bone disease in uremia. Actualités néphrologiques Jean Hamburger 95-128. [Ref.]
Download Provisional PDF Here
Article Information
Article Type: Research Article
Citation: Mahamat Abderraman G, Ka EF, Cisse MM, Lemrabott AT, Faye M, et al. (2015) Evaluation of Phospho-calcic Profile of Dakar Chronic Hemodialysis and Comparison with KDIGO Recommendations Int J Nephrol and Kidney Failure, Volume1.1: http:// dx.doi.org/10.16966/ijnkf.101
Copyright: ©2015 Mahamat Abderraman G. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
SCI FORSCHEN JOURNALS
All Sci Forschen Journals are Open Access
