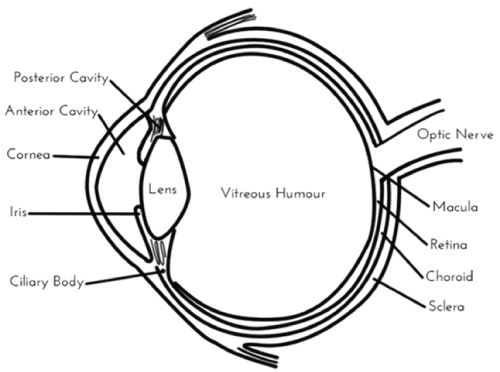
Figure 1: A diagram of the eye. The retina, vitreoushumour, and choroid are found in the posterior segment.

Shannon J Kelly Kathleen Halasz Vijaykumar Sutariya
Department of Pharmaceutical Sciences, College of Pharmacy, University of South Florida, Tampa, FL 33612, USA*Corresponding author: Vijaykumar Sutariya, Department of Pharmaceutical sciences, College of Pharmacy, University of South Florida 12901 Bruce B Downs Blvd MDC 30 Tampa, FL 33612- 4749, USA, Tel: 813-974-1401; Fax: 813-974-9890; E-mail: vsutariy@health.usf.edu
Age-related macular degeneration (AMD) and diabetic retinopathy (DR) are two of the leading causes of vision loss in people over the age of 40 in the United States [1]. Each of these conditions affects the posterior segment of the eye, which consists of the retina, vitreoushumour, and choroid (Figure 1) [2]. There are two types of AMD, dry and wet. Dry AMD results from long term choroidal ischemia, or a restriction of blood flow in the choroid of the eye [3]. Wet AMD is caused by scarring and bleeding into the macular resulting from choroidal neovascularization (CNV) [4]. Bleeding from new abnormal blood vessels in the vitreoushumour and retina indicates the development of DR [5]. The role of inflammation in AMD has been confirmed by the presence of macrophages, lymphocytes, and other inflammatory cells in the choroid [6].

Figure 1: A diagram of the eye. The retina, vitreoushumour, and choroid are found in the posterior segment.
It has been well-documented that the inflammation and angiogenesis that are characteristic of AMD and DR are caused by an increased expression of vascular endothelial growth factor (VEGF) in the eye [7- 9]. The suppression of VEGF [10-12] and amelioration of the resulting angiogenesis [13] are the current therapeutic strategies for posterior segment ocular diseases. However, VEGF is far from the only factor which can lead to pathological angiogenesis. It has been reported that the hypoxia induced factor 1α (HIF-1α) induces the over expression of VEGF and other proangiogenic factors that play key roles in ocular conditions [14,15]. The inhibition of HIF-1α may therefore prove to be a suitable therapeutic target for posterior segment ocular diseases like AMD and DR.
Several drugs have been identified as HIF-1α inhibitors including ritonavir, doxorubicin, digoxin, and honokiol (Figure 2).
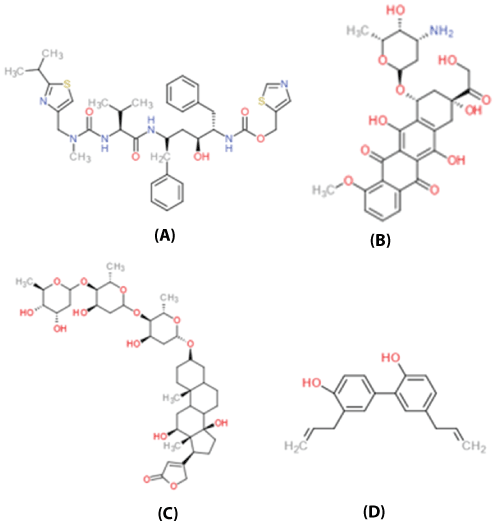
Figure 2: The molecular structures of the HIF-1α inhibitors (A) ritonavir, (B) doxorubicin, (C) digoxin, and (D) honokiol
Ritonavir (Figure 2a) is a well-documented HIV-1 protease inhibitor [16-18]. The antiangiogenic effects of ritonavir in the treatment of Kaposi sarcoma and head and neck carcinoma have since been reported [19,20]. Vadlapatla et al [21] reported a concentration-dependent decrease in VEGF secretion in ARPE-19 and D407 cells in the presence of ritonavir. This demonstrated that ritonavir inhibits HIF-1α mediated VEGF expression in retinal pigment epithelial (RPE) cells [21]. The nontoxic nature of this treatment [21] indicates that ritonavir could serve as a promising treatment for angiogenesis in posterior segment ocular diseases.
Doxorubicin (DOX) (Figure 2b) is an anthracycline antibiotic that has been used in the treatment of various cancers, including leukemia, breast cancer, ovarian cancer, lymphomas, and glioblastomas [22,23]. More recently, it has been demonstrated that doxorubicin prevents the up regulation of many proangiogenic factors, including VEGF. Iwase et al. [24] reported that a 10µg intravitreal injection of DOX reduced CNV in mice. DOX does not actually reduce the amount of HIF-1 present at the therapeutic site. Instead, DOX acts by blocking the binding of HIF-1 to DNA. Thus, it follows that DOX could serve as a possible therapy for ocular conditions resulting from angiogenesis. However, a long elimination half-life and nonspecific cell cytotoxicity have made DOX treatments disadvantageous [23,24]. Iwase et al [24] noted the development of precipitation on the retinal surface, likely resulting from the poor aqueous solubility of DOX. Studies have shown that the encapsulation of DOX by polymeric nano particles can reduce the cytotoxicity of DOX treatments and deliver a sustained release of drug [22-24].
Digoxin (Figure 2c) is a digitalis derivative that is commonly used to treat heart arrhythmia, heart failure, and atrial fibrillation. It has recently been reported that digoxin has the ability to inhibit HIF-1α protein synthesis [25]. Yoshida et al [26] reported that digoxin inhibits HIF-1α expression and reduces mRNA expression levels in mice. They further reported that a 100ng intravitreal injection of digoxin reduced retinal neovascularization in mice by 70% [26]. This potency suggests that digoxin could serve as a viable treatment for a multitude of ocular diseases caused by neovascularization, including AMD and DR.
Honokiol (Figure 2d) is a phytochemical found in the bark and cones of Magnolia plants. Honokiol has been used in traditional oriental medicine for its many therapeutic effects, including anti-inflammatory and anticancer therapies [27]. The anti-HIF [28] and anti-angiogenic [29] effects of honokiol have been described. Vavilala et al [28] found that treatment with 10µM honokiol produced a significant reduction of HIF-1α, HIF- 2α, HIF-1β, and VEGF in D407, HT29, HEK293, and ARPE-19 cell lines [28,29]. The group later published a comparison of the efficacy of honokiol on HIF suppression in ARPE-19 cells to that of doxorubicin and digoxin [30]. They concluded that honokiol was the most effective and least toxic of these three drugs. These studies suggest that honokiol offers potential for the treatment of ocular conditions caused by HIF-1α - mediated angiogenic factors.
It is well known that that inflammation and angiogenesis that causes such ocular conditions as AMD and DR is caused by an excessive production of VEGF in the eye. Current treatment strategies aim at suppressing VEGF secretion. VEGF and many other factors responsible for pathological angiogenesis are mediated by the hypoxia induced factor HIF-1α. By inhibiting HIF-1α, the excretion of these other factors will be reduced in addition to VEGF. Ritonavir, doxorubicin, digoxin, and honokiol have exhibited the ability to inhibit HIF-1α, thereby reducing the secretion of these proangiogenic factors. These HIF-1α inhibitors show potential in the treatment of ocular neovascularization. A sustained release delivery system, such as nanoparticles or thermo reversible hydrogels, could prove advantageous for treatment by reducing the required frequency of injection [2]. This is beneficial as the frequent intravitreal injections required for many current therapies can result in increased intraocular pressure, retinal detachment, and hemorrhaging. Based on the positive effects of HIF-1α inhibitors ritonavir, doxorubicin, digoxin, and honokiol, the potential of HIF-1α inhibitors in ocular therapy looks promising.
Download Provisional PDF Here
Article Type: Short Communication
Citation: Kelly SJ, Halasz K, Sutariya V (2017) HIF- 1α Inhibitors for the Treatment of Posterior Segment Ocular Diseases. Int J Nanomed Nanosurg 3(1): doi http://dx.doi.org/10.16966/2470-3206.120
Copyright: © 2017 Kelly SJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access