Abstract
Photodynamic therapy (PDT), a minimally invasive treatment modality for cancer, involving the formation of reactive oxygen species (ROS) and subsequently eradication of the tumorous cells, has received a great attention. However, its oxygen-dependent nature to certain degree limits PDT from achieving satisfactory outcomes in solid tumors because of the frequent hypoxia circumstances. To alleviate hypoxia, efforts have been made through diversified strategies, e.g., delivery of ecdemic oxygen and intracellular replenishment of oxygen during PDT. In this mini review, we further elaborate on the details of these strategies and corresponding mechanisms for better therapy efficacy.
Introduction
In recognition of its potentials for noninvasive and spatio-temporally controllable treatment of cancers, light-based therapy has been continuously explored, mainly including photodynamic therapy (PDT) [1]. and photo thermal therapy (PTT) [2]. Considering the limited therapeutic confinement, as well as the therapeutic resistance during PTT, resulting from hyperthermia-induced expression of heat shock proteins (HSPs) [3,4]. PDT, instead is seemingly a superior photo-assisted modality. During PDT, energy is transferred from photons to molecular oxygen via light-activated photosensitizers to form singlet oxygen (1O2 ) a highly reactive oxygen species (ROS) [5] which induces cell apoptosis, necrosis as well as autophagy through oxidization of lipid and proteins, damage of organelles and cell membrane [6,7]. Ongoing research on PDT is currently dominated by the 1O2 -producing type II photodynamic reactions.
Considerable efforts have been made to improve the therapeutic efficiency with the booming nanotechnology [8]. Given that light irradiation is essential to activate photosensitizers, photon energy was amplified with the aid of localized surface plasmon resonance of noble metal nanomaterials such as gold Nanoparticles [9,10]. Due to short lifespan of singlet oxygen and its consequent limited diffusion [11]. photosensitizer-loaded nanovehicles were accordingly designed to target mitochondria, an organelle primarily responsible for production of 1O2 and more importantly for cell apoptosis [12,13]. Meanwhile, to reduce the resistance of cancer cells to 1O2 cytotoxicity, 1O2 scavenger glutathione (GSH) was inactivated by copper-mediated oxidation [14].
Even with the improved photo-activation and 1O2 -sensitive toxicity, hypoxia is still a big dilemma, which makes PDT self-limited in the long run. This could be attributed to the native hypoxia and secondary low oxygen saturation caused by PDT. Intrinsically, disorganized vasculature of most solid tumors could create regions of severe hypoxia [15]. PDT is therefore, hampered by insufficient availability of molecular oxygen. Unfortunately, PDT itself could aggravate the oxygen shortage because of the continuous consumption of intracellular molecular oxygen for producing 1O2 . Furthermore, PDT is also responsible for vascular damage, which diminishes the blood circulation through tumors [16]. In this case, inadequate oxygen supplement is further deteriorated along with the PDT treatment. Dramatic PDT-induced hypoxia was confirmed in both hypoxic tumor xenografts and low to non-hypoxic ones [17]. Thus, fractional PDT (intermittent light exposure) [18] and hyperbaric oxygen inhalation [19] have been accordingly proposed to address the hypoxia scenario. However, intermittent PDT virtually extends the unpleasant treating period; side effects of hyperbaric oxygen therapy cannot be neglected. To this end, the need for better solutions in order to elevate the oxygen level, especially in solid tumorous tissues, has motivated the endeavors to seek alternatives for substantially alleviating hypoxia along with amplification of PDT efficacy. Considerable progress has been made in particular relation to oxygen elevation.
Strategies to Enrich Oxygen Supply
To better address the need of decreased hypoxia and increased oxygenation, several potential strategies, including enhanced distant oxygen delivery and local oxygen supply, have been investigated.
Transportation of ecdemic oxygen
As a straightforward solution, several studies have verified the possibility of improving oxygen transportation directly into the tumorous region. Biologically, blood circulation is responsible for O2 supply throughout the body, including the tumor tissues. In recognition, “Hot Spring” bath was employed to regulate the overall body temperature for properly accelerating the blood circulation in order to increase O2 supply [20]. During in vivo PDT with mouse breast cancer xenografts, tumor tissue oxygen saturation raised 52% along with the body temperature increase from 37º C to 43º C. Meanwhile, the photosensitization reaction rate of Chlorin e6 (Ce6) was also 20% higher at 43° C than that at 37° C, yielding an impetus to ROS generation in addition to elevated O2 supply. On the other hand, perfusion with exogenously supplied O2 is also believed to be able to effectively circumvent hypoxia. It is well recognized that hemoglobin is the essential O2 carrier in red blood cells. Thanks to its intrinsic nature of reversible binding of oxygen [21], hemoglobin was explored to be incorporated into nanomaterials for oxygen self-sufficient PDT. Wang et al. [22] demonstrated the capability of oxyhemoglobin in oxygen supply and protoporphyrin IX co-loaded red blood cell-derived vesicles (HbO2 &PpIX@RDVs) as a promoter for efficient PDT. In 2016, the attempt toward biomimetic artificial red cells (ARCs) was reported by Cai’s group (Figure 1) [23]. In their report, the typical ARC was composed of biocompatible lipid shell and poly (D, L-lactic-co-glycolic acid) (PLGA) core, where hemoglobin was cooperated with photosensitizer indocyanine green (ICG) to form an Hb/ICG complex. Upon light irradiation at 808 nm, ICG-loaded ARCs (I-ARCs) led to 9.5 times higher ROS formation than those deoxy-ICG nanoparticles. The capability of hemoglobin of reversibly combining with oxygen was well maintained in the ARCs. Two days after the initial intratumoral I-ARC injection and NIR exposure, necrotic cellular debris and severe damage to tumor tissue were observed as verified by histological analysis of the tissue cross-sections stained with hematoxylin and eosin (H&E) (Figure 1c). In both reports, a steady release of oxygen has been achieved.
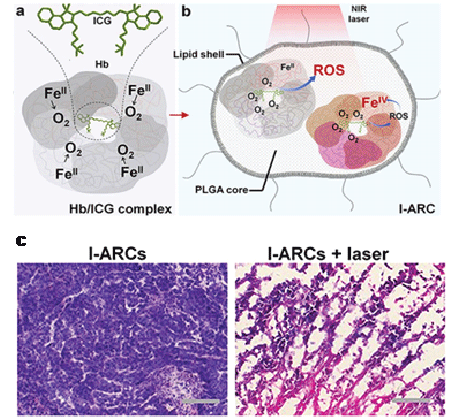
Figure 1: Schematic illustration of (a) Hb/ICG complex and (b) hemoglobin-encapsulated artificial red cell (I-ARC); (c) Optical microscopic images of the cross-sections of tumor tissues without (left) and with (right) laser treatment after staining with hematoxylin and eosin (H&E) [23]. Reproduced with permission.
Taking into account that hemoglobin, as a protein, might be vulnerable and susceptible to conformation alteration during the chemical modification procedures, the utility of other alternatives such as solutions/materials with considerable oxygen storage capacity would be a better choice.
As the blood substitute [24], perfluorocarbon (PFC) is marked for its completely fluorinated carbon skeleton, in which the high electronegativity turns out to be able to increase oxygen affinity and facilitate the solubility and diffusivity as well as exhibit longer 1O2 lifetime than water. Learned from radiation therapy (RT), which shares partial similarities to PDT in terms of oxygen dependence [25,26], PFC was accordingly engaged in PDT in several recent studies. In a novel approach of oxygen self-enriched PDT, photosensitizer IR780 was encapsulated in a monolayer of lipid nanosystem while having the O2 -containing perfluorohexane droplet as the core (Figure 2a) [27]. As shown in TUNEL assays, a larger number of apoptotic cells were observed in the photodynamic treated tumors with the PFC-assisted O2 supply, which was also confirmed by H&E staining. In this system, the sustained oxygen supply enabled a long-lasting PDT with the full achievement of the therapeutic efficacy of IR780. It exhibited a superior inhibition for tumor growth to traditional PDT even with a singledose intravenous injection, which promisingly minimized the bio-safety concerns in consideration of the low dosage needed in total. In another interesting attempt, Que et al. [28] embedded pentafluorophenyl group and porphyrin into PEG-based amphiphilic copolymers (Figure 2b), which then assembled them into nanomicelles. A higher content of pentafluorophenyl segment led to a high oxygen solubility and diffusivity and consequently promoted the formation of cytotoxic1O2 . Furthermore, a physical process that could trap oxygen in the polymer bubbles and then release it in a thermal-responsive manner was also demonstrated. In the work from Huang et al. [29] on monocyte-mediated therapy, Ce6 and superparamagnetic iron oxide nanoparticle (SPION)-loaded polymersomes were saturated with oxygen through pure oxygen purging during lyophilization and then followed by re-hydration under oxygen atmosphere. Release of O2 was triggered upon its volumetric expansion in response to the local heat as a result of high frequency magnetic field applied on the SPIONs.
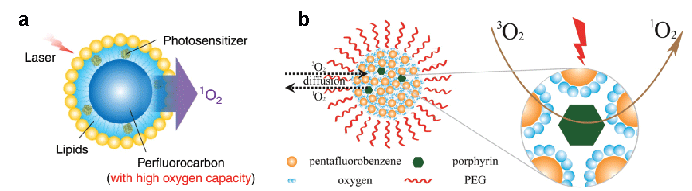
Figure 2: Schematic illustration of mechanisms for (a) O2 delivery via PFC-lipid core/shell nanostructure and (b) enhancement of PDT efficacy by fluorinated domains. Reproduced with permission [27-28].
“Excavation” of local oxygen source
From different perspective, access to the local chemical source of oxygen is another conceivable strategy, mostly focusing on intracellular hydrogen peroxide and water. As a hallmark of malignant cancerous cells, excessive amount of H2O2 is often detected [30], which could serve as both oxygen source and the trigger to initiate PDT, according to Chen et al. [31]. When H2O2 diffused across the PLGA shell and then into the aqueous core containing catalase, the decomposition mechanism was “switched on”. The outbreak of gaseous oxygen was able to rupture the PLGA shell to release the encapsulated photosensitizer, methylene blue (MB), which was subsequently activated upon laser irradiation (Figure 3a). Meanwhile, the oxygen gas in turn provides supplement for the oxygen consumption by PDT. Interestingly, molecular oxygen could be further elevated with the presence of oxygen donating manganese oxide (MnO2 ) [32]. Typically, MnO2 , under the neutral pH, simply works as a catalyst, similar to the catalase mentioned above. In the case of an acidic pH within the malignant cancerous cells, besides catalyzing H2O2 decomposition MnO2 itself was reduced to Mn2+, which could yield even greater amount of O2 with the same amount of H2O2 (Figure 3b). Thus, in tumor tissues not only O2 consumption was compensated but also oxygenated/ deoxygenated hemoglobin ratio and vascular saturated O2 were increased when compared to the saline-treated control groups.

Figure 3: Schematic illustration on (a) the mechanism of H2O2 -controllable release of photosensitizer and O2 for improved PDT, (b) balanced chemical equations for H2O2 decomposition with the presence of MnO2 in neutral (pH 7.4) and acidic (pH 5.5) solutions, and (c) light-driven water splitting by carbon dot-doped C3N4 . Reproduced with permission [31-33].
In recognition of its abundance in the living body, water could be used as an endless source for oxygen. Inspired by the solar-enabled water splitting in the exploratory efforts for clean and sustainable energy, 2D nanomaterial of carbon nitride (C3N4) with exceptional electronic properties and high biocompatibility has been introduced into the PDT nanosystems [33]. In this endeavor, C3N4 nanosheets were decorated with carbon dots for sufficient red light absorption (at 630 nm) in order to split the intracellular water (Figure 3c). Indeed, enhanced oxygen and downregulated hypoxia-associated protein levels including hypoxia-inducible factor-α (HIF-α) and carbonic anhydrase 9 (CA 9)were observed. The extra O2 produced by water splitting was converted into cytotoxic singlet oxygen (1O2 ). As a consequence, distinctively improved prognosis was achieved. Compared to control groups, inhibited tumor metastasis in liver and lung were observed with oxygen self-sufficient groups, based on both H&E staining and immunofluorescence analyses.
Conclusion and Future Prospects
In this mini-review, we have summarized several possible strategies to overcome the hypoxia hindrance confronted in cancer PDT. As illustrated, ecdemic molecular oxygen could be trapped via biological, biomimetic or physical mechanisms, and then delivered into solid tumor with the assistance of nanostructures. On the other hand, intracellularly catalyzed decomposition or oxidation of H2O2 as well as the water splitting could supply the PDT with the continuous local source of oxygen. Either strategy has demonstrated its promise and potentials in elevating the oxygen levels for enhanced PDT.
Despite the exciting progress, additional issues need the attention in better application of above strategies for future PDT. The most important concern is from the excessive oxygen supply. Unlike cancerous cells, healthy cells are more susceptible to excessive oxygen without over-expressed the antioxidants. Hence, for ecdemic oxygen supply, the possible leakage of oxygen during transportation should be cautiously prevented, particularly for those via the intravenous injection. On the other hand, the rate for intracellular release of oxygen should be precisely controlled to avoid the massive burst, which may drive excessive oxygen to the neighboring healthy tissues. In terms of local oxygen source excavation, promoted motion of nanomotors is presumable to make the best of native H2O2 or H2O in tumor tissue. Meanwhile, it may also expedite the diffusion of generated 1O2 . In the water splitting strategy, photonic energy is partially used for the production of O2 . As a result, less energy is available for photosensitizer activation, which is, leading to less 1O2 generation. The energy shortage could become pronounced in tumors located in the deep tissues. In this regard, an on-site laser source (e.g. rechargeable persistent phosphors) might be a good solution [34].
To better address the oxygen limitation, tactics that could extricate PDT from oxygen-dependence would be more appealing for future photochemistry-based cancer therapy. Type-I PDT is believed to generate cytotoxic radicals while less relying on the tissue oxygen [35]. It offers a better choice when featured sensitizers for photo-induced electron transfer and hydrogen abstraction are well designed. Although 1O2 - donating endoperoxide therapy [36] is technically not the same as PDT, it may be considered as one of preferable or eventual alternatives for nextgeneration of PDT.
During cancer therapy, however, the phenotypic heterogeneity among individuals and even within the same neoplastic mass [37], recently termed as molecular pathological epidemiology (MPE), could lead to a significant disparity in treatment efficiency as a result to varying responses to 1O2 . In this regard, the establishment of 3D tumor models with patient’s own cells would serve as an effective prescreening platform [38], enabling the provision of personalized protocol to individuals for treatment precision. Clearly, significant efforts are still required for PDT in achieving the optimal treatment efficiency and specificity.
Article Information
Article Type: Mini Review
Citation: Zhang B, Wang Z, Wang H (2016) Photodynamic Therapy for Solid Tumor Therapy: No Longer Restricted by Hypoxia. Int J Nanomed Nanosurg 2(4): doi http://dx.doi.org/10.16966/2470- 3206.118
Copyright: © 2016 Zhang B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
Received date: 29 Sep 2016
Accepted date: 24 Oct 2016
Published date: 28 Oct 2016




