Abstract
Cancer forms exhibiting poor prognosis have been extensively researched for therapeutic solutions. One of the conventional modes of
treatment, chemotherapy shows inadequacy in its methodology due to imminent side-effects and acquired drug-resistance by cancer cells.
However, advancements in nanotechnology have opened new frontiers to significantly alleviate collateral damage caused by current treatments
via innovative delivery techniques, eliminating pitfalls encountered in conventional treatments. Properties like reduced drug-clearance and
increased dose efficacy by the enhanced permeability and retention effect deem nanoparticles suitable for this application. Optimization of
size, surface charge and surface modifications have provided nanoparticles with stealth properties capable of evading immune responses, thus
deeming them as excellent carriers of chemotherapeutic agents. Biocompatible and biodegradable forms of polymers enhance the bioavailability
of chemotherapeutic agents, and permit a sustained and time-dependent release of drugs which is a characteristic of their composition, thereby
providing a controlled therapeutic approach. Studies conducted in vitro and animal models have also demonstrated a synergism in cytotoxicity
given the mechanism of action of anticancer drugs when administered in combination providing promising results. Combination therapy has also
shown implications in overcoming multiple-drug resistance, which can however be subdued by the adaptable nature of tumor microenvironment.
Surface modifications with targeting moieties can therefore feasibly increase nanoparticle uptake by specific receptor-ligand interactions,
increasing dose efficacy which can seemingly overcome drug-resistance. This article reviews recent trends and investigations in employing
polymeric nanoparticles for effectively delivering combination chemotherapy, and modifications in delivery parameters enhancing dose efficacy,
thus validating the potential in this approach for anticancer treatment.
Keywords
Nanomedicine; Drug delivery; Oncology; Multi-drug resistance; Controlled release; Enhanced permeability and retention; Receptor
mediated endocytosis; Biopolymers; Biocompatibility; Biodegradability
Background
Many prevalent forms of malignant cancers have accounted for high
mortality rates for the past few decades. Although substantial development
is achieved in chemotherapeutic treatments, effective diagnosis and
treatment of cancer involves careful consideration of the heterogenous
tumor microenvironment, an area that is relatively poorly understood.
The tumor microenvironment is created in response to the progression
of a neoplastic disease state which arises through what is known as the
“hallmarks of cancer”. The key hallmarks include sustained proliferative
signaling, evasion of growth suppressors, resistance to cell death and
subsequent cell immortality, evasion and in some cases even recruitment
of the immune system, angiogenesis or blood vessel formation, invasion
and metastasis. Tumor cell heterogeneity is a result of inflammation
and genomic instability, where a single advantageous mutation exists
without repair and further mutations in cell divisions are permitted in
the cancerous cell population. The characteristics of reprogrammed
cell energy metabolism and evasion of the immune system are also key
factors in the formation of the tumor cell microenvironment. The genetic
alterations, cell abnormalities, complexities and heterogeneous nature can
lead to multi-drug resistance (MDR) by limited access to tumors and nonspecific
targeting when using single drugs [1].
Commercially available chemotherapeutic agents with established
anticancer properties are now being explored using nanotechnology.
The advent of nanomedicine has reduced the obstacles encountered in
conventional treatments by decreasing drug-related toxicity and MDR,
while improving plasma half-life, bioavailability and biodistribution of
drugs [2-4]. Nanoparticles facilitate a sustained, controlled and targeted
drug delivery method which enhances dose efficacy and reduces side
effects. An added advantage is the increase in the drug-uptake by enhanced
permeability and retention (EPR) effect which takes advantage of the
imperfect tumor vasculature. Moreover, actively targeting nanoparticles
to malignant cells via receptor-specific interactions can demonstrate an
increased uptake due to receptor mediated endocytosis (RME) (Figure 1).
As illustrated in figure 1, non-targeted nanoparticles may be phagocytozed
by certain cells or may act as drug depots in the extracellular space and
release drug which then may diffuse across cell membranes to the cytosol
where most drug targets reside. Well-designed, targeted, polymeric
nanomedicines can be internalized via RME and then undergo endosomal
escape thereby avoiding destruction in lysosomes due to the harsh
environment including low pH and enzymatic degradation. Endosomal
escape can thus help release drugs into the cytosol and improve treatment
efficacy especially for diseases like cancer.
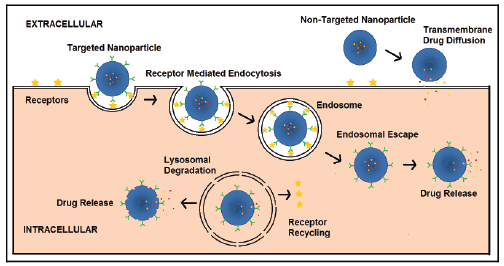
Figure 1: Scheme illustrating differences in drug release and cellular
localization for targeted and non-targeted PEGylated polymeric
nanoparticles. Targeted nanoparticles are taken up by Receptor mediated
Endocytosis while non-targeted nanoparticles may release drug in
the extracellular space which then diffuses across the cell membrane.
Recent research has employed combination therapy to target various
metabolic and physiological characteristics in cancer cells in order to
reduce drug resistance, however pharmacokinetics vary and inconsistent
drug uptake within tumor cells and suboptimal drug combination at
tumor sites occurs [1-3,5-7]. The maximum tolerated dose (MTD) does
not factor in drug synergisms which are affected by drug dosing and
scheduling of multiple drugs [2]. The overall therapeutic effects are greater
than the additive effect of the individual drugs in synergistic combination
drug therapy [2-4,8-12]. Co-delivered drugs can target similar or different
pathways and function synergistically to increase efficacy and selectively
[4]. The co-encapsulation of drugs with different physicochemical
properties, drug loading ratios and sequential drug-release in nanoparticles
therefore proves useful in combination therapy [2-4,11-13]. Application
of combination therapy via free-drug regimens in clinical trials has
exhibited a treatment effect advocating the use of combination drugs
over single-drug regimens. Moreover with nanotechnology, considering
the degradation characteristics of polymeric nanocarriers and significant
differences in release patterns of multiple drugs have shown enhanced
synergism in combination therapies [12,14].
One of the commonly used polymers, poly (lactic-co-glycolic acid)
(PLGA) is an FDA-approved polymer employed in many biomedical
applications due to its excellent biocompatible and malleable properties
[15]. Its biocompatible nature prolongs its blood circulation, thereby
increasing the plasma half-life of encapsulated drugs in addition to its
advantages including high-drug loading capacity favoring hydrophobic
drugs, subsequent increased intracellular delivery of drugs and solid matrix
protection of the drugs against degradation. Evasion of the mononuclear
phagocytic system (MPS) utilizing diblock polymers or polyethylene glycol
modified (PEGylated) forms such as PLGA-PEG further enhance the systemic
circulation time, allowing a greater uptake of chemotherapeutic agents.
The individual block component ratios can be modified to suit a particular
application thereby allowing control over the rate of polymer degradation,
and hence a desired drug-release profile [15]. Polymeric nanocarriers allow
for conjugation of targeting ligands capable of actively enhancing uptake in
malignant cells, thereby exploiting the characteristic leaky tumor vasculature
allowing selective extravasations of conjugated nanoparticles and longer
retention time due to poor lymphatic drainage [1].
Investigations in in vitro cultures and animal models have determined
aspects of nanoformulations capable of enhancing the efficacy of
treatments in clinical settings. Dose efficacy estimations by cytotoxicity
assays and assessment of drug-release profiles provide an improved
understanding of the treatment mechanism, thus evaluating the potential
of polymeric nanoparticles in anticancer applications.
Particle size and surface characteristics are primary features influencing
the bioavailabilty of encapsulated chemotherapeutic agents to tumor sites.
Recent in vitro and animal model studies have highlighted the importance
of nanoparticle sizes less than 200 nm accounting for longer systemic
circulation time, lower cytotoxicity, greater stability and favorable uptake
by the EPR effect [11-13,16-20]. Nanoformulations with relatively larger
sizes (<500 nm) are prone to systemic clearance and have demonstrated
the need for suitable surface modifications to potentially evade MPS
recognition [21]. Conjugation of chemotherapeutic agents and targeting
ligands to the polymer backbone has been implemented as an effective
approach in optimizing actively-targeted nanoformulations [9]. Size
determination of modified nanoparticles by Dynamic Light Scattering
(DLS) and Transmission Electron Microscopy (TEM) have indicated
minor fluctuations in sizes post surface modifications and drug loading,
while still retaining their sizes in the ideal range [18,20]. Surface charge
greatly regulates cellular interaction of nanoparticles, with cationic
nanoparticles demonstrating a higher cellular uptake as compared
to anionic particles [18,21,22]. However, in the case of polymeric
nanoparticles positive surface charge has been associated with increased
cytotoxicity in vivo. Suitable surface modification in several studies to
shield cationic groups; for example, the use of PEG has demonstrated
reduction in cytotoxic effects due to cationic charges [16]. Particles with a
low anionic charge (-20 mV to -40 mV) would present as ideal candidates
for in vivo application therefore striking a balance between charge related
cytotoxicity and uptake [2,18,21,22].
The interdependency of polymer composition and particle
characteristics discussed above has been crucial in the development
of nanoformulations. Hydrophobic and hydrophilic natures of
polymer components influence the drug loading capacity and facilitate
conjugation of targeting moieties and chemotherapeutic agents [2], with
hydrophobic drugs such as paclitaxel, curcumin, cisplatin and docetaxel
displaying high encapsulation in hydrophobic polymer cores via selfassembly
over hydrophilic drugs such as gemcitabine, anthracycline and
irinotecan. However, alternative approaches such as surface conjugation
of drugs and modifications in polymer composition can enable greater
encapsulation of such hydrophilic agents [9,18-20,22]. The important
aspect in combination chemotherapy however is the synergistic effect
of the delivered chemotherapeutic agents. Traditionally limited by poor
bioavailability and short plasma-life [8,23], nano-formulations delivering
combination chemotherapy provide an improved, controlled and sustained
release, thereby showing synergistic effects. Combination therapy has
allowed for dynamic re-networking of signalling mechanisms permitting
a time and pH-dependent release of drugs providing synergistic effect in
vitro cultures [3,23].
In work presented by Muntimadugu et al., a classic example of synergism
due to combination therapy is showcased via targeted PLGA nanoparticles
for breast cancer treatment (Figures 2A and 2B). Fairly monodisperse
particles with a polydispersity index (PDI) less than 0.3 were synthesized
with minor increase in sizes post paclitaxel (PTX) and salinomycin (SLM)
loading and surface modification, while still maintaining sizes under 150
nm. Particles displayed a positive surface charge of +50 mV conferred
by diododecyltrimethylammonium bromide (DMAB), hyaluronic
acid (HA) ligand-modified surface and loading of chemotherapeutic
agents. Although a positive surface charge contributed towards a
higher particle uptake, this study did not evaluate the cationic chargerelated
cytotoxicity that would have presented as an issue in vivo.
Nanoparticles (NPs) displayed high encapsulation of paclitaxel and
salinomycin individually given their hydrophobic nature, however
attempts to co-encapsulate these agents in a single carrier significantly
reduced encapsulation of only paclitaxel. Combination of PTX NPs and
HA-targeted SLM NPs demonstrated highest synergistic effect favored
by HA targeting and sustained release of these chemotherapeutic agents.
SLM-NPs and targeted SLM-HA-NPs showed a complete drug-release over
a period of 60 days, with a longer release time in PTX-NPs considering the
hydrophobic-hydrophobic interactions of drugs and polymer components
(Figure 2C). Nanoformulations demonstrated up to a two fold increase
in cytotoxicity when compared to their free-drug counterparts in MCF-
7 cells, and a four-fold increase in SLM-HA-NP targeted formulation
(Figure 2D). This combination therapy, even though not optimized for
co-encapsulation of chemotherapeutic agents, exemplifies the potential of
combination therapy using polymeric nanocarriers.
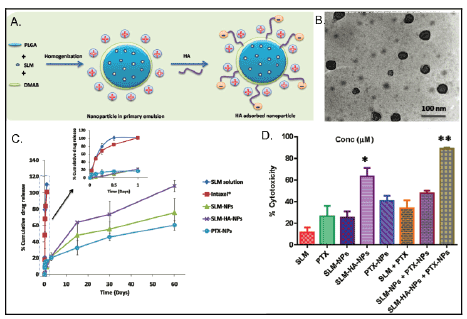
Figure 2: Comparison of Various Paclitaxel and Salinomycin Delivery Vehicles. a) Illustration of SLM-HA-NP. In the presence of diododecyltrimethylammonium
bromide (DMAB), the nanoparticle surface becomes positively charged. The addition of hyaluronic acid partially neutralizes the positive charge
of the nanoparticle. b) TEM imaging of nanoparticles confirming their size and spherical shape. c) In vitro drug release study. Complete release of SLM
and PTX was achieved after 60 days. d) % cytotoxicity of different SLM and PTX formulations, including free drugs, nanoencapsulation, targeted nanoencapsulation,
and dual-loaded targeted and non-targeted nanoparticles after 48 h of exposure. Cytotoxicity was determined by MTT assay. Adapted
from Muntimadugu et al. [18]
The true value of using polymeric nanoparticles for combination
therapy in cancer can be assessed in vivo using relevant models of the
disease. A study conducted by Shin, et al. illustrated a polymeric micelle
based delivery method that employed the use of three medications.
Paclitaxel, 17-AAG, and rapamycin were conjugated to a PEG-bpolylactic
acid (PLA) copolymer. Since these three drugs are hydrophobic,
polymeric micelle conjugation decreases the hydrophobicity of the
treatment. It was determined in this study that the three-in-one loading
method could deliver these cytotoxic agents safely and effectively to the
tumor site. The evidence supporting this included a high tolerance of the
drug in FVB albino mice. This study determined that the half-life of the
drug was between 1-15 hours, illustrating safe decomposition of the drug
in vivo [24].
In a study by Wang et al. prodrugs of baicalein (BCL) and paclitaxel
(PTX) which contained dual-targeted ligands of folic acid (FA) and
hyaluronic acid (HA) were utilized in a prodrug-based nano-drug
delivery system (P-N-DDS). Results of this study have been reproduced
in figure 3. The P-N-DDS combines two polymer-drug conjugates which
each carry single drug agents (Figure 3A). Valine and lysine are used as
connections between the drug and the ligands to obtain the prodrug.
Amino acid linkers versus poly-ethylene-glycol (PEG) provide the
advantage of weaker bonds that allow for faster drug-release. PEG has
been associated with lower efficacy than the drug alone. This study used
nanoprecipitation to make NPs which had BCL and PTX in the inner core
of a PLGA polymer-based NP. These nanoparticles were characterized by
TEM (Figure 3B). The synergistic, antitumor effects of combined drug
therapy were assessed in vitro using human lung cancer A549 cells (Figure
3C) and drug-resistant lung cancer A549/PTX cells (Figure 3D). CD44/
CD168 receptors and folate receptors over expressed on lung cancer cells
which provided a targeted mechanism for NP drug delivery with HA and
FA binding to these receptors, respectively. In vivo studies were performed
in mice with A549/PTX drug resistant human lung cancer xenograft to
determine antitumor efficiency and systemic toxicity. The statistical
significance of the results was tested using the two-tailed t-test or oneway
analysis of variance, whereby a P value less than 0.05 was considered
statistically significant.
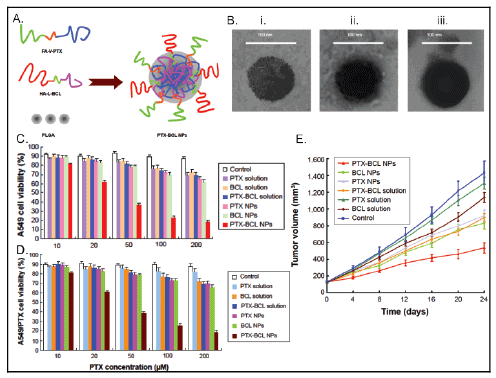
Figure 3: Synthesis, cytotoxicity and effects on tumor volume of paclitaxel and baicalein combination nanoformulation. a) The targeted PTX-BCL NPs synthesis
approach is shown using HA and FA targeting ligands which yielded greater than 86% encapsulation for both drugs. b) NP sizes less than 100 nm were obtained by
TEM imaging which was favorable for the application. The cytotoxicity of combination NPs was higher than free-drug and single-drug NPs in c) A549 cells and d)
paclitaxel-resistant A549 cells observed. e) The lowest tumor growth rate was observed in PTX-BCL NPs compared to free-drug formulations or single-drug NPs. The
PTX/BCL ratio was ⅕ (w/w) in PTX-BCL NPs and free drug PTX-BCL solution.
PTX-BCL NPs had an average size, PDI, and zeta potential of 91.8
± 2.3 nm, 0.1 ± 0.03, and 3.3 ± 0.6 mV, respectively. PTX and BCL in
the PTX-BCL NPs had an EE value of 91% and 88%, respectively. PDI
showed uniformity in the NPs, while the positive zeta potential allowed
for increased residence time; cell penetration and internalization of the
NPs. High EE values were desirable for in vitro cytotoxicity and in vivo
antitumor efficacy. Cytotoxicity assays were performed in vitro using
the MTT assay. PTX-BCL NPs showed greater cytotoxicity in A549 cells
than other NP formulations or free-drug solution (P<0.05). PTX NPs
and BCL NPs also showed greater cytotoxicity than PTX-BCL solution.
Combination therapy results for both types of cells in vitro showed a
pronounced synergistic effect of PTX-BCL when using PTX: BCL ratios
of 1:5 and 1:2. A ratio of 1:5 was used in vivo in PTX-BCL NPs. In vivo
studies demonstrated that PTX NPs were less cytotoxic than BCL NPs,
possibly due to the suppression of PTX MDR by BCL. Both PTX-BCL
solution and PTX-BCL NPs showed better antitumoral effects over
PTX alone (Figure 3E). Tumor growth was significantly inhibited by NP
formulations compared to free drug solutions. Tumor inhibition was
more successful using drug loaded NPs versus free drug solutions. Tumor
regression resulted from the use of PTX-BCL NPs as well. Body weight
loss was used as an indicator for systemic toxicity (data not shown). No
significant weight loss was found with the use of PTX-BCL NPs, while
toxicity was observed in PTX solution and PTX-BCL solution treated
specimens.
The study noted that future experiments would need to determine
optimal doses for anticancer effects and minimal systemic toxicity, as well
as applications of the procedure to other types of cancer [14]. The use
of positively charged NPs must be taken into consideration, however. A
positive zeta potential, although useful in cell membrane penetration and
drug uptake, may be hazardous in vivo [25]. Cationic NPs are not currently
approved by the FDA for clinical use due their enhanced cytotoxicity
characteristics. There are known destructive effects on cell membranes
caused by cationic NPs, in addition to dose and time dependent hemolytic
anemia and pulmonary side effects [25]. Zeta potentials falling between ±
20 mV are desirable and infer electrical stability of the NPs, while small
zeta potentials may result in coagulated NPs and less stability [25].
Conclusions
Polymeric nanocarriers have certain advantages over other modes of
drug delivery like free and conjugated drugs. Nanoencapsulation provides
a more efficient and stable delivery mechanism of chemotherapeutic
agents, especially if those agents are hydrophobic. The ability to fabricate
polymeric nanoparticles with sizes under 200 nm and a negative surface
charge favors them as carriers in comparison to other encapsulation
methods such as liposomes and dendrimers. Dual-loaded particles
convey much higher efficacy than combined free-drug solutions as seen
in the study by Wang et al. [14]. By controlling particle size, charge, and
conjugating targeted ligands to the particle, a drug that evades clearance
with tumor target specificity can be created.
As seen in the Muntimadugu et al. [18] and Parhi et al. [2] studies, the
addition of a targeted ligand to the nanoparticle surface greatly enhances
drug delivery by a factor of 2-fold compared to non-targeted nanoparticles.
By choosing which ligands to incorporate into the nanoparticle, the
researcher can create a custom delivery mechanism to match the cancer
type, ensuring tumor cell specificity. This greater specificity, high blood
plasma stability, longer drug-release time, and clearance evasion, offers an
improved treatment over chemotherapy alone.
Future Directions
Polymeric nanoparticles have created many alternative methods of
drug loading, as seen in the sections above. Due to the availability of
many different types of biopolymers, various medications can be loaded
into nanoparticles and then effectively released at the desired target site.
Finally, by loading polymeric compounds with multiple drugs to target
multiple hallmarks of cancer, more effective treatment methods can be
discovered. These nanoparticles can be targeted to deliver drugs at a
desired location.
While targeted therapy using NPs is promising in treating neoplastic
diseases, there are acquired traits, or hallmarks, that may cause the drug
to be ineffective over time because of the complex adaptation of cancerous
cells to cellular environmental stresses. Transitory clinical responses have
been followed by relapses of disease-state due to the targeting of one
capability of the cell and subsequent enabling of another. An example
given by Weinberg et al. is the efficacy of vascular endothelial growth
factor (VEGF) receptor targeting and antiangiogenesis drugs. Cancer
cells may reduce their dependence on one mechanism of adaptation
and acquire a new trait, thus increasing the likelihood of drug resistance
in the future. Inhibition of angiogenesis has been shown to reduce the
size of tumors and cause dormancy of cancer cells, however results have
been fleeting. Tumor cell adaptations such as invasion and metastasis
may be amplified in response to anti-angiogenesis [26]. Zhao et al. [12]
also showed that dual-drug loading of doxorubicin and curcumin by a
pH sensitive prodrug allowed high drug loading capacity and release of
drug contents within the tumor cell cytoplasma and nuclei. A Schiff ’s
base linker that breaks in the acidic environment of the tumor allows for
targeted therapy in tumor cells. The aforementioned studies target tumor
cells in diverse approaches but similarly strive to achieve ratiometric
controls of drug concentrations in order to provide cytotoxic effects on
targeted tumor tissue via synergistic drug co-delivery. Biopolymers could
be used in similar fashion to achieve controlled release of multiple drugs
targeting different hallmarks of cancer for effective cancer treatment.
The future of successful NP use in treating cancer lies in the
understanding of genetic factors such as spontaneous and induced
mutations (such as in virus associated cancers), the subsequent DNA
proofreading and apoptotic signaling pathways, epigenetic markers,
micro-RNAs, antibody therapy use in combination with chemotherapy
drugs, and heterotypic interactions of different cell types within the body
during the various stages of neoplastic disease. The phenotypic differences
between normal and cancer cells along with the use of the hallmark traits
of cancer will continue to bring more questions and answers as research
evolves and methods of detection change [26].
Polymeric nanoparticles are one of the most studied organic strategies
for nanomedicine, especially for combination therapy against cancer.
Tremendous interest lies in the potential of polymeric nanoparticles to
revolutionize modern, personalized cancer medicine. To determine the
ideal polymeric nanoplatform for more effective and targeted delivery
of drugs, particle size, morphology, polymeric material choice, and
processing techniques are all going to remain major research areas of
interest. Applications of polymeric nanoparticles include drug delivery
via techniques such as conjugation and entrapment of drugs or prodrugs,
stimuli-responsive systems, imaging contrast agents, and theranostics.
Issues of scale-up in manufacturing and poorly defined, regulatory
considerations continue to remain the major challenges in the clinical
development of polymeric nanoparticles. However, with increased
collaborations between academia and industry, learning from past
regulatory successes and the development of better in vitro and in vivo
models continued success in the field is guaranteed.
Acknowledgements
The authors acknowledge funding from the National Cancer Institute, a
part of the National Institutes of Health (Award # 4R00CA153948).




