Introduction
The use of vascular disrupting agents (VDAs) is one of the therapeutic
approaches that target already-established tumor vasculatures of solid
tumors [1]. There are two classes of VDAs, i.e., tubulin-binding VDAs
and flavonoid VDAs [1]. Tubulin-binding VDAs selectively disrupt
the cytoskeleton of proliferating tumor blood vessel endothelial cells.
Flavonoid VDAs induce apoptosis in tumor blood vessel endothelial cells.
Arsenic trioxide has been used clinically as an effective agent for acute
promyelocytic leukemia [2]. It has been reported that arsenic trioxide has
an antivascular effect on solid tumors as a tubulin-binding VDA [3] and
induces vascular shut down and necrosis in the tumor [4].
Hyperthermia is one of the approaches to cancer therapy, and is
based on the fact that cancer cells are more sensitive to heat than normal
tissues. The most commonly used heating method in the clinical setting
is capacitive heating that employs a radiofrequency (RF) electric field
[5]. The therapeutic effect of hyperthermia depends on the temperature
and the heating duration. The cell-killing mechanism of hyperthermia is
linked to activation of the immune system [6] and its efficacy increases
dramatically at temperatures above 42.5°C. The heating duration can be
shortened with each 1°C temperature increase to give an equivalent cellkilling
effect [7]. The therapeutic effect of hyperthermia can be enhanced
by combining it with radiotherapy, chemotherapy and/or immunotherapy
[8-10]. Recently, hyperthermia combined with VDA has been introduced
[11] and it has been reported that arsenic trioxide can increase tumor
thermo-sensitivity and heat-induced tumor growth delay [11].
Magnetic hyperthermia treatment (MHT) is one of the hyperthermia
treatments and employs the temperature rise of magnetic nanoparticles
(MNPs) under an alternating magnetic field (AMF). MNPs generate
heat through hysteresis loss and/or relaxational loss due to Nѐel and
Brownian relaxations when exposed to AMF [12]. Although conventional
hyperthermia treatments such as RF-capacitive heating [5] damage not
only cancer cells but also normal tissues, MHT can selectively heat tumor
cells without damaging normal tissues [13]. In order to enhance the
therapeutic effect of MHT, it is necessary to deliver and accumulate as
many MNPs as possible into the tumor tissues [12].
Magnetic particle imaging (MPI) is an imaging modality that was
introduced in 2005 [14]. MPI uses the nonlinear response of MNPs to an
external oscillating magnetic field and is capable of imaging the spatial
distribution of MNPs such as superparamagnetic iron oxide with high
sensitivity and high spatial resolution [14].
The purpose of this study was to quantitatively evaluate the tumor early
response to MHT combined with VDA in comparison with that to MHT
alone using MPI.
Materials and Methods
System for magnetic particle imaging
The details of our MPI system are described in our previous reports
[15-17]. In brief, a drive magnetic field was generated using an excitation
coil (solenoid coil 100 mm in length, 80 mm in inner diameter, and 110
mm in outer diameter). AC power was supplied to the excitation coil by a
programmable power supply (EC1000S, NF Co., Yokohama, Japan), and
was controlled using a sinusoidal wave generated by a digital function
generator (DF1906, NF Co., Yokohama, Japan). The frequency and peakto-peak
strength of the drive magnetic field were taken as 400 Hz and 20
mT, respectively [15]. The signal generated by MNPs was received by a
gradiometer coil (50 mm in length, 35 mm in inner diameter, and 40 mm
in outer diameter), and the third-harmonic signal was extracted using
a preamplifier (T-AMP03HC, Turtle Industry Co., Ibaragi, Japan) and a
lock-in amplifier (LI5640, NF Co., Yokohama, Japan). The output of the
lock-in amplifier was converted to digital data by a personal computer
connected to a multifunction data acquisition device with a universal
serial bus port (USB-6212, National Instruments Co., TX, USA). The
sampling time was taken as 10 msec. When measuring signals using the
gradiometer coil, a sample was placed 12.5 mm (i.e., one quarter of the
coil length) from the center of the gradiometer coil and the coil, including
the sample, was moved such that the center of the sample coincided
with the position of the field-free line. The selection magnetic field was
generated by two opposing neodymium magnets (Neomax Engineering
Co., Gunma, Japan). The field-free line can be generated at the center of
the two neodymium magnets.
To acquire projection data for image reconstruction, a sample in the
receiving coil was automatically rotated around the z-axis over 180° in
steps of 5° and translated in the x-direction from −16 mm to 16 mm in
steps of 1 mm using an XYZ-axes rotary stage (HPS80-50X-M5, Sigma
Koki Co., Tokyo, Japan) controlled using LabVIEW (National Instruments
Co., TX, USA). Data acquisition took about 12 min. Each projection
data set was then transformed into 64 bins by linear interpolation. Both
the inhomogeneous sensitivity of the receiving coil and feed-through
interference were corrected using the method described in [16]. Transverse
images were reconstructed from the projection data using the maximum
likelihood-expectation maximization (ML-EM) algorithm over 15
iterations, in which the initial concentration of MNPs was assumed to be
uniform [15,17]. In this study, the MPI value was defined as the pixel value
of the transverse MPI image reconstructed from the third-harmonic signals.
Apparatus for magnetic hyperthermia treatment
The details of our apparatus for MHT are described in our previous
report [18]. In brief, the coil for generating an AMF consists of 19-turned
loops (6.5 cm in diameter and 10 cm in length) of copper pipe (5 mm
in diameter) and is cooled by water to ensure constant temperature and
impedance. The coil is connected to a high-frequency power supply
(T162-5723BHE, Thamway Co., Ltd, Shizuoka, Japan) and a manualmatching
unit (T020-5723AHE, Thamway Co., Ltd, Shizuoka, Japan). The
peak amplitude of the AMF generated in the coil can be controlled by
changing the output of the power supply.
Animals and tumor model
All animal experiments were approved by the animal ethics committee
at Osaka University School of Medicine. Seven-week-old male BALB/c
mice were purchased from Charles River Laboratories Japan, Inc.
(Yokohama, Japan), and were habituated to the rearing environment for
one week before the experiment. The animals had free access to food and
water, and were kept under standard laboratory conditions of 23°C room
temperature and around 50% humidity.
Colon-26 (a mouse cell line derived from rectal cancer) cells (Riken
BioResource Center, Ibaragi, Japan) were cultured in RPMI-1640 medium
(Mediatech Inc., VA, USA) supplemented with 10% fetal bovine serum
(FBS) (Biowest, Nuaillé, France) and 1% Penicillin-Streptomycin (Nacalai
Tesque Inc., Kyoto, Japan). All cultures were incubated in a humidified
atmosphere containing 5% CO2
at 37°C. Cells were trypsinized with
0.25% trypsin in ethylenediaminetetraacetic acid (EDTA) (Nacalai Tesque
Inc., Kyoto, Japan) and resuspended in phosphate-buffered saline (PBS) at
1 × 106
cells/100 µL.
The cells (1 × 106
cells) were implanted into the backs of 8-weekold
mice on the same day and under the same conditions. During the
implantation, the mice were anesthetized by intraperitoneal injection of
pentobarbital sodium (Somnopentyl, Kyoritsu Seiyaku Co., Tokyo, Japan)
at a dose of 0.012 mL/g body weight (BW) (10-fold dilution).
Vascular disrupting agent
In this study, arsenic trioxide (Trisenox®, Nippon Shinyaku Co., Ltd.,
Kyoto, Japan) was used as VDA. When investigating the effect of VDA,
mice were intraperitoneally injected with Trisenox® at a single dose of 4
mg/kg BW.
Magnetic nanoparticles
In this study, Resovist® (FUJIFILM RI Pharma Co., Ltd., Tokyo, Japan)
was used as the source of MNPs, because it is commercially available and
has been approved for clinical use in Japan. Resovist® consists of MNPs
(maghemite, γ-Fe2
O3
) coated with carboxydextran. It is an organ-specific
contrast agent for magnetic resonance imaging (MRI), used especially
for the detection of hepatocellular carcinoma and liver metastasis [18].
According to Biederer et al. [19], the mean and standard deviation of the
particle size of Resovist® are 15.2 nm and 3.21 nm, respectively.
Tumor blood flow measurement
Tumor blood flow was measured using a laser Doppler flow meter
(FLO-N1, OMEGAWAVE Inc., Tokyo, Japan). When measuring the
tumor blood flow, each tumor-bearing mouse was fixed on a board with
surgical tape under anesthesia and a probe was set approximately 5 mm
above the tumor surface. Continuous recordings were initiated 10 min
before the intraperitoneal injection of VDA (n=4) or PBS (n=3) to obtain
the baseline blood flow and lasted for 2 hours.
Study protocol
Figure 1 shows the time schedule for data acquisition in the present
study. When the tumor volume had grown to approximately 100 mm3
,
mice were divided into two groups. One group consisted of mice treated
with MHT in combination with VDA (MHT+VDA group, n=12), whereas
the other group consisted of mice treated with MHT alone (MHT group,
n=11). For comparison, we also used the data of the mice without MHT
and VDA as control (control group, n=9). In the MHT+VDA group, VDA
was intraperitoneally injected in a single dose of 4 mg/kg BW. Two hours
after the injection of VDA, Resovist® (0.2 mL of stock solution diluted in
PBS) with an iron (Fe) concentration of 250 mM (14.0 mg Fe/mL) was
directly injected into the tumor under anesthesia. Fifteen min after the
injection of Resovist®, each mouse was placed in the holder and set in the
coil for generating an AMF. MHT was performed by applying an AMF at a
frequency of 600 kHz and peak amplitude of 3.5 kA/m [18] for 20 min. In
the MHT group, only MHT was performed without the injection of VDA.
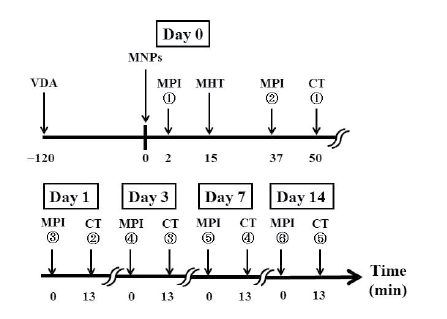
Figure 1: Time schedule for data acquisition in the present study. VDA:
vascular disrupting agent, MNPs: magnetic nanoparticles, MPI: magnetic
particle imaging, CT: X-ray computed tomography, and MHT: magnetic
hyperthermia treatment. Note that we used arsenic trioxide (Trisenox®)
as the VDA and injected it into mice intraperitoneally, whereas we used
Resovist® as the source of MNPs and injected it directly into the tumor.
Each tumor-bearing mouse was scanned 6 times using our MPI scanner
[15]; immediately before MHT, immediately after MHT, and 1, 3, 7, and
14 days after MHT (Figure 1). After the MPI studies, X-ray CT images
were obtained using a 4-row multi-slice CT scanner (Asteion, Toshiba
Medical Systems Co., Tochigi, Japan) with a tube voltage of 120 kV, a tube
current of 210 mA, and a slice thickness of 0.5 mm. The MPI image was
co-registered to the X-ray CT image to confirm the spatial distribution of
Resovist® in the tumor-bearing mouse using the method described in [20].
It should be noted that the X-ray CT image after the first MPI study was
substituted by that obtained after the second MPI study.
Histological study
Separately from the above studies, mice were purchased for histological
studies and were implanted with colon-26 cells in the same manner as
described above. The tumor-bearing mice were sacrificed and the tumors
were removed immediately after MHT, and 3, 7, and 14 days after
MHT (n=3 each). The tumor tissues were fixed in a 7.5% formaldehyde
neutral buffered solution (Sigma-Aldrich Japan Co., Ltd., Tokyo, Japan),
dehydrated, and embedded in paraffin. They were then sectioned at a
thickness of 3 μm on a microtome and stained with hematoxylin and
eosin (H&E). The histological images were acquired with a microscope
(ECLIPSE80i, NIKON Co. Ltd., Tokyo, Japan) at a magnification of 20
and imaging software (NIS-Elements D, NIKON Co. Ltd., Tokyo, Japan).
Data analysis
The dimensions of the tumors in all mice were measured with a caliper
every day until 14 days after MHT. The tumor volume (V) was calculated
from V = (π/6 ) × Lx × Ly × Lz, where Lx, Ly, and Lz represent the vertical
diameter, the horizontal diameter, and the height in mm, respectively. The
relative tumor volume growth (RTVG) was also calculated from (V−V0
)/ V0
, where V0
represents the tumor volume immediately before MHT. In this
study, the RTVG value was used as an indicator of the therapeutic effect
of MHT+VDA or MHT.
After the MPI studies, we drew a region of interest (ROI) on the tumor
in the MPI image and calculated the average, maximum, and total MPI
values and the number of pixels within the ROI by taking the threshold
value for extracting the contour of the tumor as 40% of the maximum MPI
value within the ROI. The total MPI value is equal to the product of the
average MPI value and the number of pixels.
Statistical analysis
The tumor blood flow, RTVG value, average, maximum, and total
MPI values and the number of pixels within the ROI were expressed
as the mean ± standard error (SE). The one-way analysis of variance
(ANOVA) was used for comparison among three groups and statistical
significance was determined by Tukey’s multiple comparison tests. The
Student’s t test or Welch’s t test (two-tailed) was used for comparisons
between two groups. A P value less than 0.05 was considered statistically
significant.
Results
Figure 2 shows the time courses of the tumor blood flow measured
using a laser Doppler flow meter in the mice injected with VDA (●,
n=4) and PBS (■, n=3). In the mice injected with PBS, the tumor blood
flow slightly increased up to approximately 10 min and plateaued for
approximately 30 min. It then decreased gradually up to 70 min and
became stable thereafter. In the mice injected with VDA, the tumor blood
flow decreased up to approximately 30 min and then increased gradually
to the initial blood flow level approximately 100 min after VDA
injection. The tumor blood flow in the mice injected with VDA was
significantly lower than that in the mice injected with PBS 15 to 50 min
after the injection of VDA or PBS.
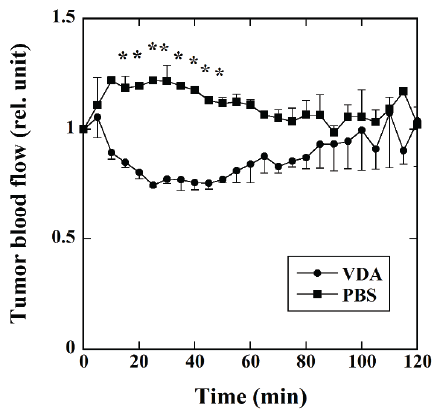
Figure 2: Time courses of the tumor blood flow measured using a laser
Doppler flow meter in mice injected with VDA (●, n=4) and phosphatebuffered
saline (PBS) (■, n=3). The blood flow values 5 to 120 min after
the injection of VDA or PBS was normalized by the average blood flow
value for 10 min before the injection. *: P<0.05.
Figure 3 shows the time courses of the RTVG values in the MHT+VDA
(●, n=12), MHT (■, n=11), and the control groups (▲, n=9). The RTVG
value in the MHT+VDA group was significantly lower than that in the
MHT group 1 and 2 days after MHT. The RTVG value in the MHT+VDA
group was also significantly lower than that in the control group 2 to 4
days after MHT.
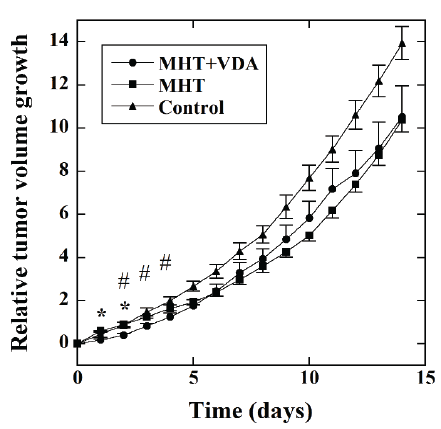
Figure 3:Time courses of the relative tumor volume growth (RTVG)
values in the MHT+VDA (●, n=12), MHT (■, n=11), and control groups
(▲, n=9). The mice in the MHT+VDA and MHT groups were treated with
MHT combined with VDA and MHT alone, respectively, whereas those in
the control group were not treated with MHT and VDA. *: P<0.05 between
the MHT+VDA and MHT groups and #: P<0.05 between the MHT+VDA
and control groups.
Figure 4 shows the MPI images superimposed on the X-ray CT images
immediately before MHT (a), immediately after MHT (b), and 1 day (c), 3
days (d), 7 days (e), and 14 days after MHT (f). The upper and lower panels
show those in the MHT+VDA and MHT groups, respectively. As shown
in figure 4, the MPI value tended to decrease and the spatial distribution
of MNPs changed with time in both groups. It was visually confirmed that
the MPI value in the MHT+VDA group tended to be higher than that in
the MHT group 1 to 14 days after MHT.
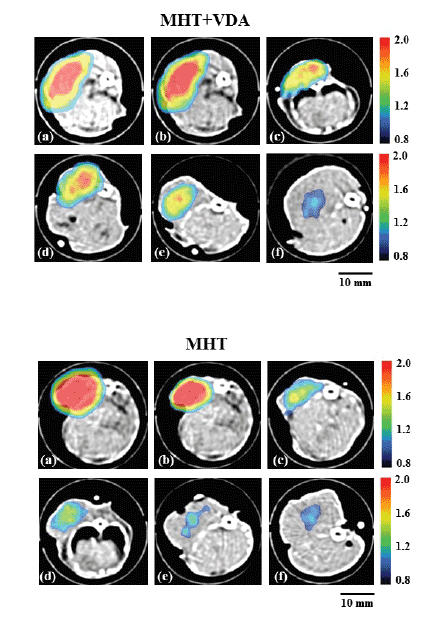
Figure 4: MPI images superimposed on X-ray CT images in the
MHT+VDA (upper panels) and MHT groups (lower panels) immediately
before MHT (a), immediately after MHT (b), and 1 day (c), 3 days (d), 7
days (e), and 14 days after MHT (f). Scale bar = 10 mm.
Figure 5 shows the temporal changes of the average MPI value (a),
maximum MPI value (b), number of pixels within the ROI (c), and total
MPI value (d). The closed and open bars show those in the MHT+VDA
(n=12) and MHT groups (n=11), respectively. The average and maximum
MPI values in the MHT+VDA group were significantly higher than those
in the MHT group 3 and 7 days after MHT. The average and maximum
MPI values in the MHT+VDA group also tended to be higher than those
in the MHT group 14 days after MHT. In these cases, the P values were
0.065 and 0.075 for the average and maximum MPI values, respectively.
Although the number of pixels within the ROI tended to increase with
time in both groups, and the total MPI value in the MHT+VDA group
tended to be higher than that in the MHT group 3 to 14 days after MHT,
they did not reach statistical significance due to large scattering of the data.
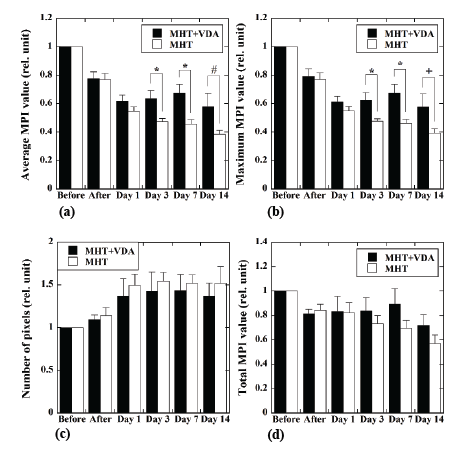
Figure 5: Temporal changes of the average MPI value (a), maximum
MPI value (b), number of pixels within the region of interest (ROI) (c),
and total MPI value (d). The closed and open bars represent cases for
the MHT+VDA (n=12) and MHT groups (n=11), respectively. Each bar
and error bar represent the mean and standard error, respectively. The
values immediately after MHT, and 1, 3, 7, and 14 days after MHT were
normalized by those immediately before MHT. *: P<0.05, #: P=0.065,
and +: P=0.075.
Figure 6 shows typical H&E stain images in the MHT+VDA (left
column) and MHT groups (right column) immediately after MHT (top
row), and 3 days (second row), 7 days (third row), and 14 days after MHT
(bottom row). As shown in figure 6, the necrotic area (shown by N) in the
MHT+VDA group was larger than that in the MHT group in all cases.
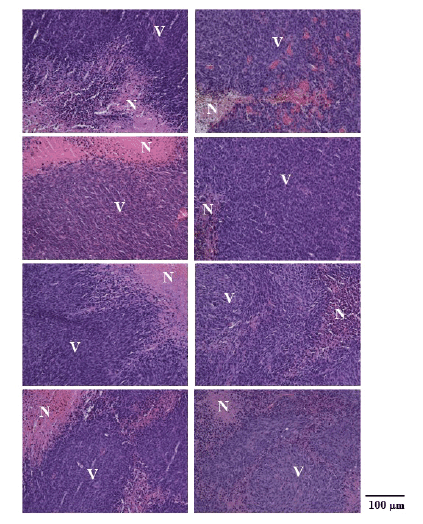
Figure 6: Hematoxylin and eosin (H&E) stain images in the MHT+VDA
(left column) and MHT groups (right column) immediately after MHT (top
row), and 3 days (second row), 7 days (third row), and 14 days after MHT
(bottom row). Magnification, X20. N: necrotic area and V: viable area.
Scale bar=100 µm.
Discussion
In this study, we investigated the tumor response to MHT+VDA in
comparison with that to MHT alone by quantitatively evaluating the
temporal change of the MNPs in the tumors of tumor-bearing mice
using MPI. We also investigated the synergistic effect of VDA on MHT
in terms of RTVG. As previously described, we used Trisenox® at a dose
of 4 mg/kg BW as VDA and Resovist® with an iron concentration of 250
mM as the source of MNPs. In this study, we injected Trisenox® into each
tumor-bearing mouse intraperitoneally only once, whereas we injected
Resovist® directly into the tumor and MHT was performed only once
for each mouse. This is mainly because the purpose of this study was to
quantitatively evaluate the tumor response to MHT+VDA in comparison
with that to MHT alone using MPI, rather than to search for a method for
enhancing the therapeutic effect of MHT.
As shown in figure 3, the RTVG value in the MHT+VDA group was
significantly lower than that in the MHT group 1 and 2 days after MHT.
It was also significantly lower than the RTVG value in the control group
2 to 4 days after MHT. However, there was no significant difference in
the RTVG value between the MHT and control groups. In our previous
studies [20,21], when an AMF with the same frequency and peak
amplitude as those in this study was applied to the tumor injected with
250 mM Resovist® for 20 min, the temperature in the tumor rose to around
41°C. It has been reported that mild hyperthermia at approximately 40°C
decreases the induction of cytotoxicity and endoplasmic reticulum (ER)
stress and rather protects cells against ER stress-induced apoptosis [22]. In
contrast, arsenic trioxide is useful for decreasing tumor blood perfusion
and inhibiting the heat dissipation and can increase tumor thermosensitivity
at temperatures as low as 41.5°C [11]. Thus, we can expect a
synergistic effect of VDA in mild hyperthermia when combined with MHT.
In our histological study, the necrotic area in the MHT+VDA group
was larger than that in the MHT group (Figure 6), indicating that VDA is
useful for enhancing the necrosis of the tumor. Thus, our results (Figure
6) suggest that MHT combined with VDA is more effective for delaying
the tumor growth than MHT alone. Although the therapeutic effect of
VDA was observed in terms of the tumor volume growth, this effect was
limited to the early stage after VDA injection and disappeared 3 days or
more after MHT (Figure 3). VDAs induce the disruption of abnormal
peripheral blood vessels and central necrosis of tumors [1]. As described
by Tozer et al. [23], a common feature of the vascular effects of VDAs is
the sparing of tumor cells in the peripheral rim; the tumor cells in this
region survive and proliferate because they receive oxygen and nutrients
from surrounding normal tissue. This feature of VDAs appears to be the
main reason why the effect of VDA on the tumor volume growth is limited
to the early stage after VDA injection (Figure 3).
As shown in figure 2, the tumor blood flow in the mice injected with
VDA significantly decreased in comparison with that in the mice injected
with PBS 15 to 50 min after the injection of VDA or PBS. Approximately 2
hours after the injection of VDA, however, the tumor blood flow recovered
to the initial blood flow level. In this study, MHT was started 2 hours and
15 min after the injection of VDA (Figure 1). Thus, the results shown in
figure 2 suggest that there is almost no effect of VDA on the tumor blood
perfusion during MHT. This may also be one of the reasons why the effect
of VDA on the tumor volume growth is limited to the early stage after
VDA injection (Figure 3). It might be necessary to shorten the period
between the time of VDA injection and the start time of MHT to enhance
the synergistic effect of VDA. In addition to the timing of VDA injection,
the reduction of the tumor blood flow and induction of the necrosis in
the tumor depend on the injected dose of VDA [24]. If we used Trisenox®
at a higher dose than that adopted in this study (4 mg/kg BW) and/or
injected multiple doses of Trisenox®, the therapeutic effect of VDA might
be higher than that in this study. However, it has been shown that the
injection of high-dose arsenic trioxide induces systemic side effects on the
heart, lung, spleen, liver, kidney or brain [25]. Alternatively, the inhibition
of nitric oxide synthase (NOS) induces the reduction of tumor blood flow
[26] and enhances the tumor vascular damaging effect of VDA [27]. Thus,
the combination of arsenic trioxide and a NOS inhibitor may be more
effective for improving the therapeutic effect than the use of high-dose
arsenic trioxide. These studies are currently in progress.
The MPI images superimposed on the X-ray CT images were useful
for visualizing the temporal change of MNPs in the tumor (Figure 4).
As shown in figure 4, the MPI pixel values tended to decrease and the
spatial distribution of MNPs changed with time in both the MHT+VDA
and MHT groups. In addition, the MPI pixel values in the MHT+VDA
group tended to be higher than those in the MHT group. To quantitatively
evaluate the temporal change of MNPs in the tumor, we calculated the
average, maximum, and total MPI values, and the number of pixels within
the ROI drawn on the tumor in the MPI image immediately before MHT,
immediately after MHT, and 1, 3, 7, and 14 days after MHT (Figure 5). We
previously reported an excellent linear correlation between the average
MPI value and the iron concentration of Resovist® in phantom studies
[20]. From this finding, it appears that the change in the average MPI value
corresponds to that in the amount of MNPs per voxel and the change in
the total MPI value corresponds to that in the total amount of MNPs in
the selected slice of the tumor, whereas the change in the number of pixels
corresponds to that in the distributed area of MNPs. As shown in figure
5, the average and maximum MPI values in the MHT+VDA group were
significantly higher than those in the MHT group 3 and 7 days after MHT
and the total MPI value in the MHT+VDA group tended to be higher
than that in the MHT group 3 to 14 days after MHT. These results suggest
that when VDA was used, the MNPs injected directly into the tumor
were confined to the tumor and their dispersion within the tumor was
suppressed due to the vascular disrupting effect of VDA 3 to 7 days after
MHT. The fact that VDA significantly enhances the retention of MNPs
in the tumor (Figure 5) will be useful when considering the repeated
applications of MHT to enhance its therapeutic effect.
As described above, we demonstrated that MPI allows us to
quantitatively evaluate the tumor response to MHT or MHT+VDA using
values such as the average and maximum MPI values within the ROI
(Figure 5). To the best of our knowledge, this is the first report to show that
MPI is useful for evaluating the tumor response to therapeutic agents such
as VDA. We previously reported that the average MPI value is significantly
inversely correlated with the RTVG value and will be useful for predicting
the therapeutic effect and treatment planning of MHT [20,21]. We believe
that MPI can also be applied to prediction of the therapeutic effect and
treatment planning of MHT+VDA.
Other methods for evaluating the tumor early response to VDA are
positron emission tomography (PET) and dynamic contrast-enhanced
MRI (DCE-MRI) [28,29]. Guo et al. [28] reported that the retention of
radiotracers in the tumor measured by PET was significantly enhanced
by injection of VDA 1 to 3 days after the intravenous injection of
radiotracers. Gao et al. [29] reported that the retention of gadoliniumdiethylenetriamine
pentaacetic acid (Gd-DTPA) in a tumor measured
by DCE-MRI was significantly enhanced due to the injection of VDA 6
to 24 hours after the intravenous injection of Gd-DTPA. In this study,
MNPs were directly injected into the tumor as previously described and
the retention of MNPs in the tumor was significantly enhanced due to
VDA injection 3 days after MHT and lasted up to at least 7 days after
MHT (Figures 5a and 5b). The difference between our results and those
of Guo et al. [28] or Gao et al. [29] may be mainly due to the difference
in the injection route of the agents and/or the affinity of the agents with
tumor cells. When MNPs were injected intravenously, their clearance rate
from the tumor might be faster than that when they were directly injected
into the tumor. Further studies are necessary to elucidate the reason for
the above difference.
Conclusion
We demonstrated that administration of VDA induced a reduction of
tumor blood flow and delayed the tumor volume growth at an early stage
after the injection of VDA. We also demonstrated that VDA enhanced
the retention of MNPs in the tumor 3 to 7 days after MHT. These results
suggest that VDA will be useful for enhancing the therapeutic effect of
MHT when applied repeatedly. Our results also suggest that MPI is useful
for quantitative evaluation of the tumor response to therapeutic agents
such as VDA.
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research
(Grant No. 25282131) from the Japan Society for the Promotion of
Science (JSPS). The authors are grateful to Prof. Masato Ohmi of Osaka
University for his help in measuring tumor blood flow using a laser
Doppler flow meter.
Declaration of Interest
The authors report no conflicts of interest.







