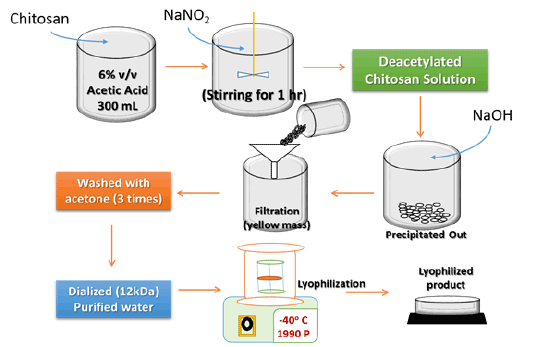
Figure 1: Schematic representation of the preparation of deacetylated chitosan.

Choudhary S V Kusum Devi* Raichur V
Department of Pharmaceutics, Al Ameen College of Pharmacy, Hosur Main Road, Near Lalbagh Main Gate Bangalore-560 027, India*Corresponding author: V Kusum Devi, Department of Pharmaceutics, Al Ameen College of Pharmacy, Hosur Main Road, Near Lalbagh Main Gate, Bangalore–560 027, India, Tel: +91 93428 59850; E-mail: drvkusumdevi4@gmail.com
Context: An attempt has been made to modify the natural polymer (chitosan) to its deacetylated form to enhance its physico-chemical properties with improved feasibility in nanoparticulate delivery systems for stability and reproducibility.
Objective: Deacetylation of chitosan was processed by removal of branched acetyl group in order to increase the polymer stability for particulate drug delivery systems with enhanced physical and process dependent properties. Alongside, preparation of deacetylated chitosan based drug loaded nanoparticles for determining its impact on formulation process and stability.
Materials and methods: Chitosan is poly [(1,4)-2-amino-2-deoxy-D-glucopyranose], a copolymers of N-acetyl-D-glucosamine and D-glucosamine (backbone chains). N-deacetylation was performed using modified alkali reduction method to get ≥99% deacetylation. Later on, nanoparticles were prepared and characterized with optimization accordingly.
Results and discussion: Implemented polymer characterization: physical parameters, solubility, degree of deacetylation, viscosity and crystallinity shown desired properties. Alongside, application based evaluations, i.e., screened and optimized preparation of nanoparticles with characterization and in vitro release behaviour.
Conclusion: Desirable changes in chitosan properties and the subsequent reproducibility of nanoparticles prepared with promising results, paves the way towards extensive research in modifications of existing natural polymers for better reproducibility at reduced costs and higher commercialization probability.
Degree of deacetylation; Polymeric Nanoparticles; Ionic gelation; Response surface plots; Probe sonication
Polymeric nanoparticles are the most promising novel drug delivery systems with enhanced drug carrying capabilities. It can incorporate even the most complex active moieties either in its core or uniformly distributed throughout the matrix [1].
The major polymers used nowadays for this system as primary carriers ranges from natural to semi-synthetic and further moves towards advanced chemically synthesized types [2,3]. But the foremost advantages of naturally obtained polymers (like chitosan, sodium alginate, etc.) are their easy availability, cost-effectiveness and most importantly their nontoxic nature. Even though, these polymers lacks purity (due to natural origin), unfavourable physico-chemical properties (like poor solubility, higher viscosity, poor flow properties. etc.) and at such level these problems ultimately leads to difficulty in getting reproducibility (during preparation of novel drug delivery forms) [4-6].
On that context, chitosan was selected in the current research purpose in order to enhance its physico-chemical properties by making changes at molecular level. Chitosan, a (1-4)-linked 2-amino-2-deoxy-D-glucan, is the N-deacetylated chitin derivative. It is a functional linear polymer that attracted mounting interest for use in drug delivery in diverse forms which is generally non-toxic and non-irritant with defined biocompatibility [7,8]. Modification in chitosan molecular chain i.e., removal of branched acetyl groups leads to exposure of amino group which can directly impart increased solubility and decreased desirable viscosity [9]. Thereby, the deacetylation of commercial chitosan retards the stability issues of the polymer which arises during formulation of particulate drug delivery systems with further enhanced physico-chemical properties. Commercial chitosan is approximately 70-75% deacetylated. Generally, the degree of deacetylation mainly depends upon alkali concentration used, temperature and time of reaction, particle size and density. A modified procedure was adopted to get ≥99% deacetylation with slightest chitosan backbone chain degradation. Henceforth, an attempt has been made to prepare completely deacetylated chitosan with superior physico-chemical properties [10,11].
Tuberculosis and its newly emerged resistant counterparts now find extensive nanoparticulate drug delivery systems emphasized research [12]. Thus, one of its sole first line drug i.e., Isoniazid (INH) was selected as a model drug for loading in characterized deacetylated chitosan nanoparticles to justify the application oriented benefits achieved.
Chitosan, 65-75% deacetylated, C1 (Himedia Pvt. Ltd., India); Glacial acetic acid (S D Finechem Pvt. Ltd., Bangalore, India); Sodium hydroxide pellets, NaOH (Finar India Pvt. Ltd. Bangalore); Sodium nitrite, NaNO2 (Finar India, Pvt. Ltd. Bangalore); Hydrochloric acid, HCl (S D Finechem Pvt. Ltd., Bangalore, India); Sodium tripolyphosphate, STPP (Sisco Research Laboratories Pvt. Ltd., India); Dialysis tube, 12-14 kDa molecular weight cut-off (Himedia Pvt. Ltd., India).
To obtain deacetylated chitosan, medium molecular weight chitosan (C1) was depolymerized as per the method explained by Moghaddamet et al. [13] with optimized modifications. Primarily, chitosan was dissolved in glacial acetic acid (6% v/v) to obtain 2% v/v chitosan solution in acetic acid. Modified deacetylated chitosan was obtained after addition of 10 mL of NaNO2 (7.0 mg/mL in distilled water) to the dissolved chitosan solution at room temperature under magnetic stirring. Nearly after an hour, the depolymerized chitosan was precipitated by raising the pH to 9.0 by adding NaOH (4 N). The white yellowish solid mass was filtered, washed with acetone practically three times, and then dissolved in a minimum volume of glacial acetic acid (0.1 N). Further, purification was carried out by subsequent dialysis against purified water. The dialyzed product was finally dried using a freeze dryer (Oper FBD-5503; Operon Inc., Korea). The yellowish lyophilized product obtained was stored at 4°C until further required (Figure 1) [14].
Organoleptic properties: Prepared deacetylated chitosan was compared visually with the commercial chitosan for changes in physical properties such as colour, odour and appearances.
Flow properties (% compressibility, angle of repose and rate of flow): Tapping method was used to measure bulk density (g/cc) and bulk volume (cc/g). Accurately weighed quantities of the samples were carefully poured into the graduated cylinder through a funnel and the bulk volume was recorded with and without tapping [15].Further, the value of bulk density was used to determine
the % compressibility (or Carr’s Index) using the formula:
$$\theta \, = \,{\tan ^{ - 1}}\,{{hight\,of\,the\,heap\,(h)} \over {radious\,of\,the\,heap\,circle\,(p)}}$$
Angle of repose (θ) was used to determine the time of flow with that of heap height of the modified chitosan in comparison to commercial chitosan. Then, the angle value (θ) was measured using the formula:
$$\% \,Copressibility\, = \,{{Tapped\,density - \,Untapped\,density} \over {Tapped\,density}} \times \,100$$
Viscosity is defined as the ability of the liquid to provide resistance to flow. Prepared chitosan solutions with 1%, 2% and 3% w/v strength of chitosan. It was measured using spindle no.21 (Brookfield Model DV-III+, Brookfield, USA) at 250 rpm (at 25°C) based on stability of the torque value.
FITR study was carried out by potassium bromide (KBr) pellet method for the modified and commercial chitosan using Shimadzu FTIR-ATR Prestige-14000, Japan. Before forming the pellet of KBr, the samples were completely dried at 100°C for one hour and then discs were pressed with KBr: sample mixture (99:1) and analysed accordingly [15,17].
X-ray diffraction patterns were determined using diffractometer equipped with a rotating target X-ray tube and a wide angle goniometer for the modified and commercial chitosan using DSC Q5000 instrument. The X-ray tube was operated at a potential of 50 kV and a current of 150 mA. The range (2θ) of scans was from 0 to 70°C and the scan speed was 2°C per minute at increments of 0.02°C. The results obtained were interpreted [15,16].
Several methods were screened for the preparation of drug loaded chitosan nanoparticles in order to define the required formulation parameters with desired particle size and polydispersity index (PDI). Henceforth, both the commercial chitosan and the modified chitosan were used to prepare nanoparticles by incorporating a model drug Isoniazid, basically by ionic gelation method with some critical standardized modifications.

Figure 1: Schematic representation of the preparation of deacetylated chitosan.
Chitosan is a charged cationic natural polymer which can interact with various cross linkers to form nanoparticles, such as: glutaraldehyde (but due to their toxicity for biological systems, especially if the unreacted cross linker remains), sodium tripolyphosphate, etc. To resolve the toxic effects of using chemical cross linkers, chitosan was ionically cross linked with multivalent anions i.e., sodium tripolyphosphate. This method has several advantages since it is a mild process resulting in nanoparticles with sizes around 200-400 nm and has been proven to encapsulate different biological and active compounds[14].
Modification (1)- ionic gelation: Primarily, 60 mg of modified deacetylated chitosan was added in 20 mL of 1% v/v acetic acid solution in distilled water. Isoniazid (INH) was added (90% w/w of chitosan added) and stirred until clear solution was obtained. A prepared solution of sodium tripolyphosphate (STPP) of 0.25% w/w as that of chitosan was added in 10 mL of distilled water drop-wise using a burette at 1200 rpm stirring speed. After the complete addition of STPP, it was further stirred for nearly 1 hour at 2000 rpm to restrict aggregation of prepared nanoparticles. Later, the prepared nanoparticles were subjected to probe sonication for 10 minutes and finally, the formulation was characterized to determine the desired properties [18,19,20].
Modification (2) - ionic gelation with pH adjustment: To further improve the desired properties, above modification was further altered. In this method, every step was performed as per the last modification, but here the pH of prepared STPP was changed from 11.0 to 3-4, in order to enhance the ionic interaction among polymer and STPP during preparation.
Modification(3) - ionic gelation with surfactant: The formulation parameters selected in Modification- (2) was satisfactory; still in order to satisfy long term stability, it was further developed. Use of surfactant is already well established in novel drug delivery platforms. Thereby, Tween 80 was selected as the typical surfactant to derive its effect in nanoparticulate formations. In this modification too, every step remained the same, except the addition of surfactant (0.1%) in the polymeric solution itself before the ionic interaction occurs.
After the screening of the above modifications, Modification (3) type was selected for the preparation of drug loaded deacetylated chitosan nanoparticles (depicted in Suppl Figure 10 of Supplementary copy).
Out of several process parameters standardized; following holds the key step determining parameters: stirring speed during cross linking (1200 rpm), stirring speed after cross-linking (2000 rpm), probe sonication time (10 + 10 minutes with 1 minute interval time). In case of standardization of formulation parameters includes: concentration of acetic acid (1% v/v) and drug concentration (90% w/w as compared to chitosan).
Optimization of the formulations were fabricated according to a Response surface plot (RSD) of Central composite design - CCD (Circumscribed type) using 32 factorial design which allows simultaneous evaluation of two formulation variables (A; concentration of chitosan and B; concentration of sodium tripolyphosphate) at three levels and their interaction. The experimental designs with corresponding formulations (outlined in table 4). The dependent variables that were selected for study were particle size (Y1 ) and polydispersibity index (PDI) (Y2 ). The selected optimized formulations were found to be 9 (nine) with 4 (four) controls.
Drug entrapment, % drug loading capacity, % yield, particle size and PDI: Lyophilized formulation containing drug equivalent to 10 mg was taken and digested with ethanolic solution (water: ethanol =7:3). Digested the homogenate and then centrifuged at 15000 rpm for 30 min. The supernatant was separated and analysed using UVVisible spectrophotometer at 263 nm to determine the drug content. % Entrapment efficiency (%EE) and % drug loading capacity (%LC) is given by [19].
$$\% EE\, = {{{\rm{total}}\,{\rm{amount }}\,{\rm{of }}\,{\rm{drug }}\, - {\rm{total }}\,{\rm{amount }}\,{\rm{of }}\,{\rm{unbound }}\,{\rm{drug}}} \over {{\rm{total }}\,{\rm{amount }}\,{\rm{of }}\,{\rm{drug}}}}$$
$$\% LC\, = \,{{{\rm{total }}\,{\rm{amount }}\,{\rm{of}}\,{\rm{drug }} - \,{\rm{total }}\,{\rm{amount }}\,{\rm{of }}\,{\rm{unbound }}\,{\rm{drug}}} \over {{\rm{total }}\,{\rm{weight }}\,{\rm{of }}\,{\rm{nanoparticles }}\,{\rm{taken}}}}$$
Dried NPs were accurately weighed and by considering the total amount of drug and excipients used for preparation of the feed solution, the yield of production (%YP) was calculated using the equation[19].
$$\% YP\, = \,{{{\rm{Nanoparticle}}\,{\rm{obtained }}\,{\rm{(NPs)}}} \over {{\rm{Total }}\,{\rm{amount }}\,{\rm{of }}\,{\rm{Drug }}\,{\rm{Excipients}}}} \times \,100$$
For finding the particle size and PDI, formulation was loaded in the cuvette and kept in the sample holder. The instrument firstly stabilized the sample at 25°C temperature for 20 sec and then scanning started for 20 consecutive runs. The result obtained with the graph was produced.
Polynomial equation was derived from the optimization of the formulations. The equation derives the effect on the selected responses (or depended variables) i.e., particle size (Y1 ) and PDI (Y2 ) when the values of independent variables, i.e., concentration of chitosan (A) and concentration of STPP (B), were altered in a sequential given rates. The equation gives the values in the form of positive or negative effect of the independent variables. After the analysis of the results, this equation may be directly used to find out the responses for any given value of independent variables. Generally, it is given as:
$${Y_1} = \, + Z \pm {Z_1}A \pm {Z_2}B \pm .........so\,on$$
$${Y_2}\, = \, + Z \pm {Z_1}A \pm {Z_2}B \pm .........so\,on$$
Where, Z, Z1 , and Z2 values are constant obtained after the optimization of experimental results[21].
Electrophoretic light scattering method was used to determine the zeta potential using instrument (Zetasizer 300 HAS- Malvern Instruments, Malvern, UK) having mobility range ± 1. 2 E-3 cm2 /V. conductivity range up to 200 mS/cm and zeta potential ± 150 mV using optics of 2 Laser (He-Ne) diodes, 658 nm, 30 mW. Peltier controller was used to control the temperature. Zeta cell was used for the sample analysis. The formulation (0.5-0.7 mL) was loaded in the cell and analysed.
Drug-excipient compatibility within the prepared formulation was investigated using FTIR spectrums of pure drug and selected formulations. Infrared spectrums of Isoniazid, physical mixture and selected formulations were recorded by Shimadzu FTIR-ATR Prestige using KBr dispersion method. The baseline correction was done using dried KBr and results were interpreted. The sample preparation was done in the same way as mentioned in section ii)-d.
Crystallinity of the pure drug was compared with that of the selected formulations to determine the change in the crystal behaviour before and after the drug and the polymer was incorporated to form the NPs. The samples were analysed in the same was as given in section ii)-e.
Similar to earlier pure drug and physical mixture compatibility testing, was measured for pure drug and selected formulations. DSC thermograms of drug and physical mixture with excipients (stored at 40°C with 75% RH for 2 months) were recorded. All the samples were placed into sealed aluminium pans and scanned at the heating rate of 10o C min-1 over the temperature range of 50-250°C. The thermograms obtained were interpreted [22].
A quantity of selected formulations i.e., Isoniazid loaded chitosan nanoparticles equivalent to 10 mg was taken in a dialysis bag (cellophane membrane, M.W. cut-off 10-12 kDa, Hi-Media, India) with 2 mL of phosphate buffer pH 7.4 buffer. The dialysis bag was then suspended in a flask containing 100 mL Phosphate buffer (pH 7.4) on a magnetic stirrer at 37 ± 0.5°C at 50 rpm. Required quantity (2 mL) of the medium was withdrawn at specific time periods (0.5, 1, 2, 4, 6, 8, 12, 18, 24, 30, 36 hrs) and same volume of fresh medium of buffer was replaced in the flask to maintain a constant volume. The withdrawn samples in the volumetric flask was made up to 25 mL using phosphate buffer (pH 7.4) and filtered. The filtrate was analyzed for drug release by measuring the absorbance at 263 nm using UV-Visible spectrophotometer (Shimadzu UV-Vis 1700). The results obtained was interpreted and plotted in the form of graphs [19].
To study the underlying mechanism of drug release, drug release data was computed by the use of following mathematical models: Zero-order kinetics, First-order kinetics and Higuchi kinetics. In order to define a model, which would represent a better fit for the formulation, drug release data was further analyzed by Korsenmeyer-Peppar equation and the results obtained was interpreted further.
i) Evaluation of both deacetylated and commercial chitosan
a) Flow properties (% compressibility, angle of repose and rate of flow) and Viscosity measurement: (Table 1)
b) FTIR Study to determine the degree of deacetylation (Figure 2, Table 2)
c) P-XRD Study to determine the crystalline properties (Figure 3)
ii) Screening of both commercial and deacetylated chitosan in preparation of drug loaded nanoparticles
Modifications were screened to get the desired particle size and in range PDI for the drug loaded deacetylated chitosan nanoparticles (Table 3).
iii) Preparation of Isoniazid loaded deacetylated chitosan nanoparticles using modified ionic gelation method
a) Experimental Design with Particle size and PDI (Table 4, Figures 4 and 5)
b) Experimental Significance of Parameters – ANOVA results (Figure 6)
iv) Characterization Studies
a) Drug entrapment, % yield, % drug loading capacity, particle size, PDI and zeta potential (Table 5)
b) Development of Polynomial Equation
The result from the optimization design i.e., the final equation in terms of actual factors:
c) Fourier Transform Infrared Spectroscopy (FTIR) study (Figure 7)
d) Powder X-ray diffraction Study (Figure 8)
e) Differential Scanning Calorimetry (DSC) study (Figure 9)
f) Invitro drug release study (Suppl Figure 11)
a) Organoleptic properties
It was found that the commercial chitosan (C1) appeared as light brown amorphous flake like structures compared to modified chitosan (C2) which appeared as yellowish white crystalline grainy powder which is nearly spherical in shape.
b) Flow properties (% compressibility, angle of repose and rate of flow)
Flow properties of modified chitosan was improved compared to the commercial chitosan as in case of % compressibility i.e., 8.86 ± 2.58% (good flow) for C2 than 20.19 ± 2.09% (poor flow) for C1, as shown in table 1
Similarly, the rate of flow was also enriched for modified chitosan (i.e., 1.98 ± 0.04 g/sec) as related to its commercial counterpart (0.92 ± 0.02 g/sec). The modified chitosan formed θ=29.41 ± 1.38°C from the base (within the range of 25-30°C for good flow) compared to the commercial chitosan which was out of the range with θ=31.99 ± 1.02°C. These data further signifies the value-added enhanced angle of repose values for the modified chitosan (refer table 1).
c) Viscosity measurement
Higher viscosity was the primary drawback of chitosan while preparing the nanoformulations. Chitosan, due to its natural origin always possesses some undissolved materials within the solution. Due to the viscosity of such strength, even through vacuum filtration the undissolved matter was not sufficiently eradicated. In case of 1% w/v solution, the viscosity of commercial chitosan was found to be 44 ± 2.0 mPas compared to much lower viscosity of modified chitosan i.e., 11.2 ± 1.8 mPas. Similarly, further observations in 2% w/v and 3% w/v solution, the viscosity was much lower for modified chitosan (20.6 ± 1.4 mPas and 26.9 ± 1.5mPas, respectively) than the commercial chitosan (71 ± 2.2 mPas and 93 ± 2.6 mPas, respectively). With viscosity, the time for solubilizing chitosan was also reduced from hours to minutes, as depicted in table 1.
d) FTIR Study to determine the degree of deacetylation
Generally, three major peaks are considered i.e., -CONH (for determining the intact and exposed amine group), -OH (to define the no. of decreased acetyl group) and –CH (as standard for the formula) as visualized in Figure 2. It was found that the commercial chitosan was 64.63% deacetylated (i.e., acetyl groups nearly 35.37%) compared to the highly deacetylated chitosan with 98.02% deacetylation (having decreased acetyl groups to 1.97%), depicted in Table 2.
e) P-XRD Study to determine the crystalline properties
P-XRD diffractogram of commercial chitosan showed merged peaks, yet some wider peaks, thus it signifies the presence of lower degree of crystallinity with fewer peaks such as, at intensities of 8.84 (273 counts), 20.02 (293 counts) and 42.58 (115 counts) respectively. In case of modified chitosan, patterns at intensities such as, 11.48 (209 counts), 19.20 (226 counts), 22.64 (273 counts), 25.34 (278 counts), 29.78 (395 counts), respectively (indicated in Figure 3); signifies that the deacetylation process increased the crystallinity of chitosan and thereby it confirms the viability of results of improved flow properties of modified chitosan with that of commercial chitosan.
The reason behind screening of such method modifications was to determine the effect of several screened parameters in method of preparation on the particle and size with respect to both commercial and modified chitosan. Modification-1, used the well-established technique but was not feasible due to unavailability of particle size near or below 200 nm. Later on, Modification-2 was inculcated to check the effect of pH (of STPP solution) over the level of interaction during formulation and found to have much higher particle size and PDI. Finally, Modification-3 was applied which was nearly similar to Modification-1 but with addition of surfactant. On commencement of this final modification, the particle size was found to be nearer to 200 nm with acceptable PDI below 0.30.
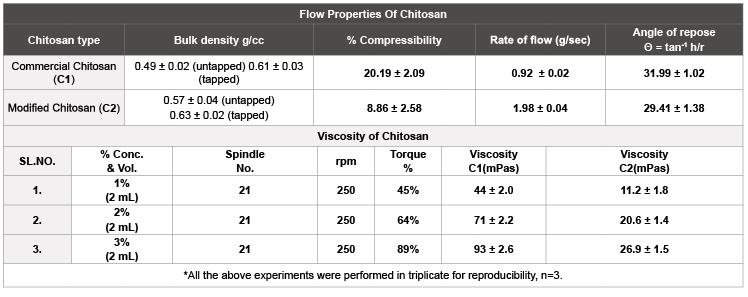
Table 1: Comparison of C1 and C2 based on flow properties.
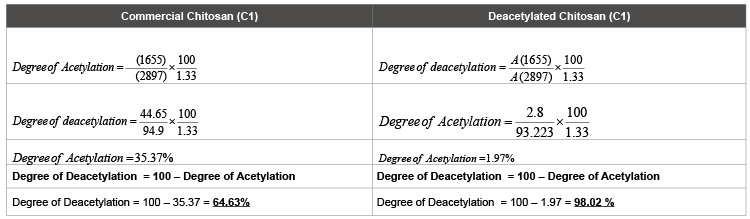
Table 2: Degree of deacetylation calculated from the FTIR data at selected wave numbers.
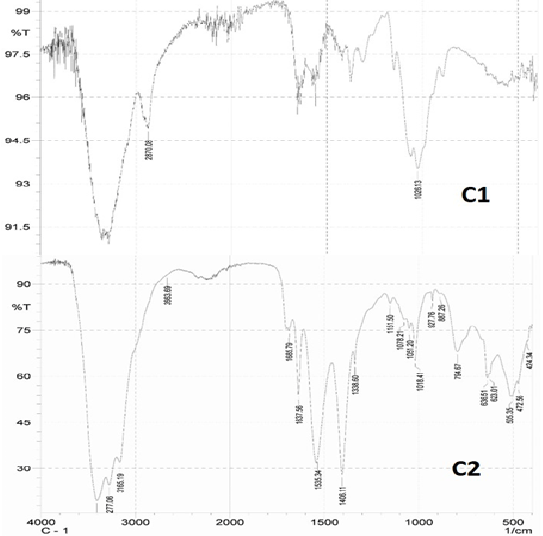
Figure 2: FTIR spectrum of Commercial (C1) and deacetylated chitosan (C2).
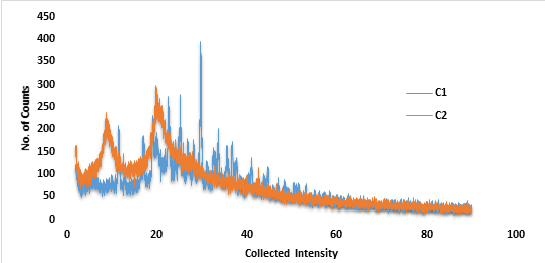
Figure 3: P-XRD plot for Commercial grade chitosan (C1) andDeacetylated chitosan (C2).
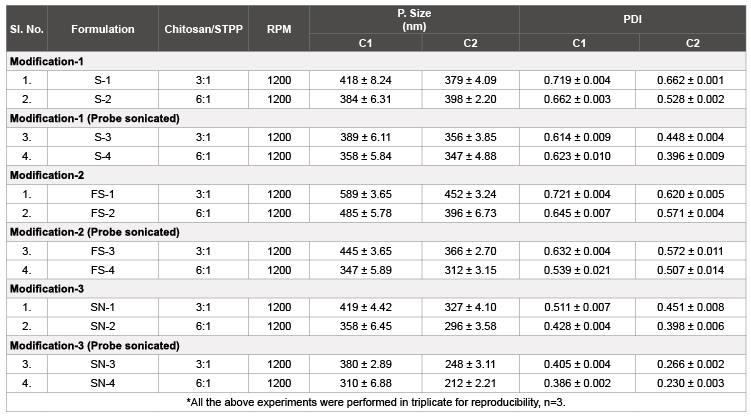
Table 3: Prepared nanoparticles using several screened modifications of ionic gelation.
Another aspect which was observed, the use of probe sonicator in all the above modifications which successively decreased the particle size and PDI and thus considered as an integral part. In the end, the primary objective of screening such parameters in order to determine the effect of modified chitosan was successfully inculcated with much faster formulation processing bonded with comparatively much lower particle size and much better PDI.
After the standardization of process and formulation parameters and selection of suitable method modification type; optimization was carried out using Response Surface plot (by employing 32 Factorial design) which gave 9 (nine) major formulations and 4(four) control formulations. The minimum range selected for chitosan was 0.9 to 1.1 i.e., 0.9 (Level -1), 1.0 (Level 0) and 1.1 (Level +1) based on ratios to STPP. Similarly, range for STPP was restricted to 0.2 to 0.30. i.e., 0.20 (Level -1), 0.25 (Level 0) and 0.30 (Level +1) based on chitosan concentration ratios. After the design was prepared, the designated formulations ranging from F-1 to F-13 were formulated and the responses were defined and analysed accordingly. Factorial design plot for particle size (Response Y1 ) was analysed in 3D plot gave following assumptions (given in Figures 4 and 5).
Based on the positive results obtained from factorial design 3D plot, the ANOVA testing was conducted and reported with following assumptions:
| Response Y2 (PDI) | :Significant model F value: 19.91 P value: 0.003 |
| Response Y1 (Particle Size) | : Significant model F value: 8.35 P value: 0.0074 |
Based on the above results, three best formulations were selected with following parameters:
| ✔ Min P. size with Min. PDI | : F-3 (Nearest value) |
| ✔ Max P. Size with Max. PDI | : F-2 (Nearest value) |
| ✔ Intermediate P. Size and In range PDI | : F-9 (Nearest value) |
a) % Drug entrapment, % drug loading capacity, % yield, particle size and PDI
The drug entrapment and loading capacity was measured using the ethanol: water (1:7). The % entrapment efficiency (%EE) was 47.1 ± 2.25 % (F-2), 46.0 ± 2.01 % (F-3) and 49.8 ± 2.45 % (F-9), respectively. In case of % loading capacity (%LC), it was reported as 14.40 ± 0.54, 10.83 ± 0.67, 17.72 ± 0.44 % (F-9), respectively (depicted in table 5).
The % practical yield for F-series formulations were found to be 122 ± 5.9 mg (89.05 % for F-2), 113 ± 6.2 mg (90.40 % for F-3) and 118 ± 5.8 mg (91.42 % for F-9) was determined.
Particle size of selected F-series formulations were found to be 241.4 ± 0.25 nm (F-2), 191.4 ± 0.24 nm (F-3) 214.9 ± 0.18 nm (F-9) with PDI as 0.215 ± 0.011, 0.206 ± 0.052 and 0.220 ± 0.088 respectively.
After optimization and AVOVA analysis, the final equation was given for responses particle size and PDI and the effect of chitosan and STPP concentration of it respectively. The equation for particle
size signifies that:
Similarly, the equation for PDI signifies that:
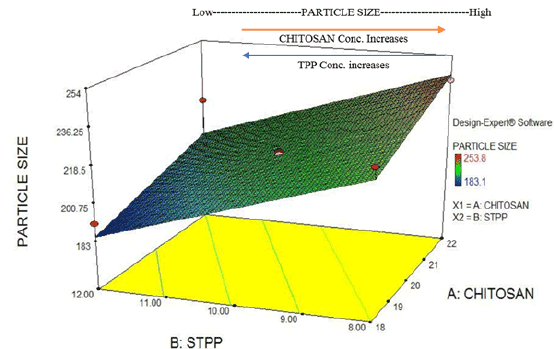
Figure 4: Factorial design plot: Particle Size (response) for drug loaded chitosan NPs.
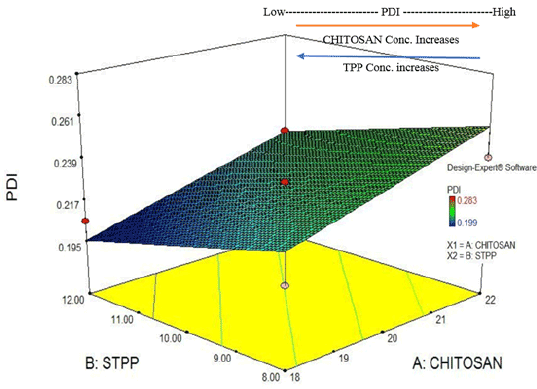
Figure 5: Factorial design plot: PDI (response) for drug loaded chitosan NPs.
Zeta potential values were determined by diluting the sample with distilled water, which was found to be +22.15 ± 1.37 mV.
Selected formulation (F-9), it was found that most of the characteristic peaks of pure drug and chitosan was either reduced in size or merged with he formulation based wider peaks, such as –C=C- (1552.70 cm-1), -C=N stretch (1633.71 cm-1)), -C=O stretch (1662.62 cm-1)), -N-H (3302.13 cm- 1 ), -C-C-C asymmetric bend (742.59 cm-1)) and –C-C-C symmetric bend (503.43 cm-1)) for pure drug (as shown in Supplementary copy Suppl Figure 12). With respect to excipients, it was found that one characteristic peak was merged with adjacent formulation peak i.e., -OH stretch (3375.12 cm-1)) and two peaks were reduced further in size i.e., -CONH (1667.77 cm-1)) and –CH stretch (2860.43 cm-1)) respectively. These changes in peak absorbance determines the probable interaction between drug and excipients within the formulation F-9 and thus signifies the formation of nanoparticles(depicted in Figure 7).
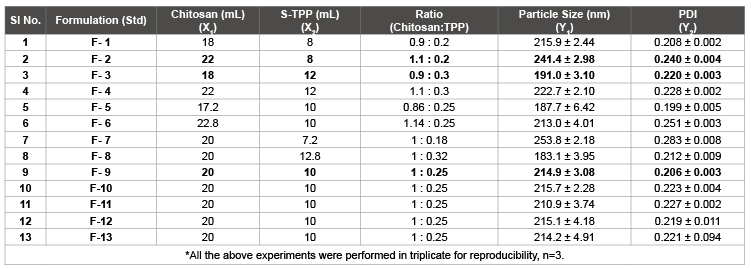
Table 4: Experimental design with responses using Surface plot of drug loaded Chitosan NPs.
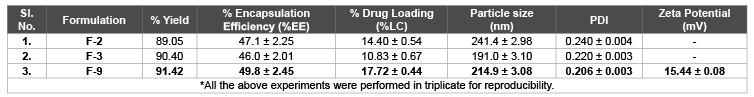
Table 5: % yield, %EE, %LC, particle size, PDI and zeta potential for selected best formulations.
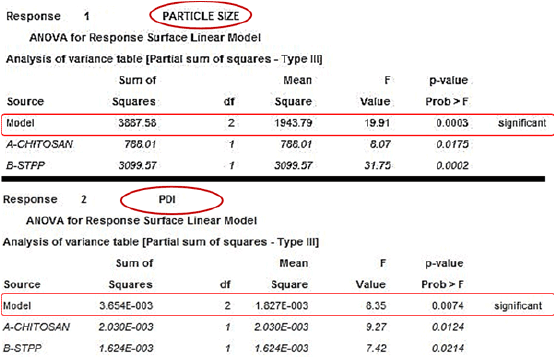
Figure 6: ANOVA table for determining the significance of particle size and PDI.
P-XRD diffractogram of formulation F-9 consists of closely merged peaks and thus signifies partial amorphous nature of the sample in comparison to pure drug and excipient diffractogram (Supplementary copy, Suppl Figure 13) and thereby a defined set of interaction was visible among the drug and the excipients to prepare nanoparticles (shown in Figure 8).
On equating the thermo grams of pure drug with formulation F-9, it was found that the values of glass transition temperature was 58°C (35°C for pure drug, Supplementary copy, Suppl Figure 14), onset of melting temperature was 139.63°C (170.70°C for pure drug, Supplementary copy, Suppl Figure 14) and melting peak was 150.34°C (175.70°C for pure drug, Supplementary copy, Suppl Figure 14) which was depicted in Figure 9. Such shifts in the thermo gram temperature based peaks clearly expresses the bonding between the drug and excipients within the formulations with decreased level of crystallinity (due to smaller onset and melting peaks) in comparison to highly crystalline (due to sharp and longer defined peaks) pure drug.
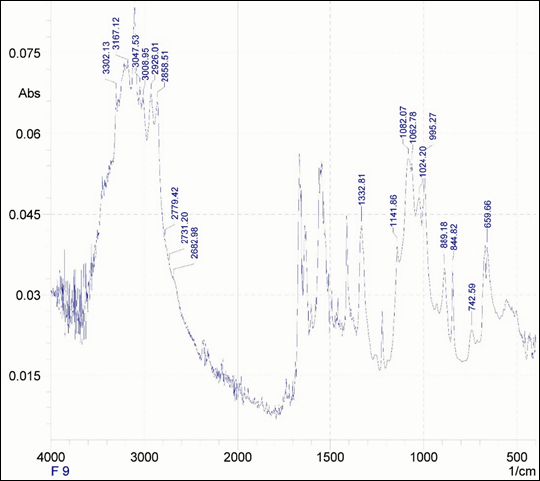
Figure 7: FTIR spectrum of prepared formulation F-9.
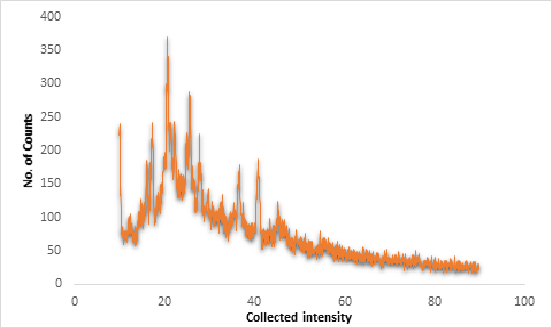
Figure 8: P-XRD diffractogram of formulation F-9.
In vitro drug release study was carried out for formulation of F-series (F2, F3 and F-9) in Potassium dihydrogen phosphate buffer (pH 7.4) and the graph was plotted between % drug release (Y-axis) vs Time in minutes (X-axis).The graph of formulation F-series showed that the release was more in case of formulation F-3 (>93.78%) compared to F-2 (87.22%) and F-9 (91.48%) given in Suppl Figure 10. Formulation F-9 showed intermediate release pattern as compared to other formulations. This implies the slight effect of particle size on in vitro drug release from the nano formulations. After such release level, model fitting (i.e., Kinetic modelling) was estimated using PCP Disso Software (version 3.0) for formulation F-9 and it showed the 1st order release with R2 value 0.9870 and k value -0.0018. The Korsmeyer-Peppas equation gave n value = 0.64146 and k value = 1.1022 (thus signifies anomalous release pattern much likely 1st order).
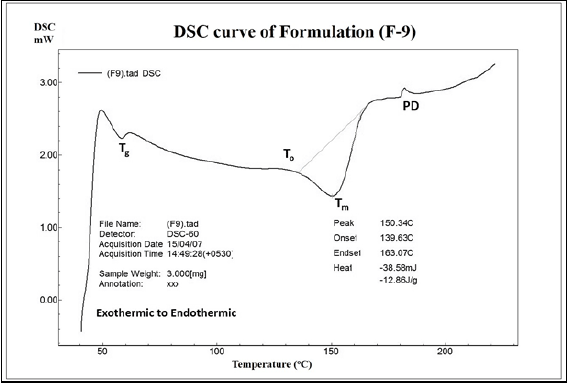
Figure 9: DSC thermogram of formulation F-9.
Commercial chitosan was modified to form deacetylated chitosan. Evaluation of physicochemical properties, such as: organoleptic properties, flow properties, viscosity, etc. showed far better enriched properties compared to commercial chitosan. Apart from that, the degree of deacetylation reached at its highest level which clearly signifies the effectiveness of the method used for deacetylation. Crystalline level was much improved for modified chitosanand thus supported the data of enhanced flow properties. With the increase in deacetylation level and crystallinity of chitosan, the solubility also enhanced to several extent.
With respect to the application part in novel drug delivery approaches, the formulation method was quite easier due to improved physicochemical properties i.e., modified chitosan. On comparison, in every method, the particle size and PDI was better in modified chitosan than its commercial counterpart with remarkable reproducibility. Based on such assumptions, the selected method was used to prepare ionized loaded deacetylated chitosan nanoparticles with application of optimization using 32 full factorial designs. Later, the evaluation of the parameters, such as particle size, PDI, % entrapment, % yield, drug-excipient compatibility studies, etc. showed promising results. In vitro drug release profile too emphasized on controlled release of the drug from the formulations. In short, the deacetylation process helped in enhancing the physicchemical parameters of chitosan, which in turn enhanced the formulation aspects. Thus, it can be concluded that the demerits of natural polymer chitosan i.e., solubility, viscosity, purity, etc. may be eradicated using the above technique which can help in the use of such modified polymer in nanoparticulate drug delivery systems with promising reproducible results and promising cost effectiveness with least toxicity due to natural origin of polymer.
I deeply acknowledge the support in procuring API by Calyx Pharmaceuticals Pvt. Ltd. A huge vote of thanks to Dr. Shobharani R. Hiremath (Principal), Al-Ameen College of Pharmacy, Bangalore; for her support in providing all the required facilities. I also heartly acknowledge the much needed encouragement provided by Mr. Mohammednadeem, Ms. Monika Pant and Mr. Ashani Basu throughout. At the end, the ultimate reason in this project’s successful completion is the selfless support from the dedicated professors & lecturers of all departments and Ms. Sabiha Banu’s team staffs.
We hereby declare that there were no conflicts of interests among the listed authors.
Download Provisional PDF Here
Aritcle Type: Research Article
Citation: Choudhary S, V Kusum Devi, Raichur V (2015) N-deacetylation and Characterization of Chitosan: Impact on Optimized Nanoparticulate Drug Delivery Systems. Int Nanomed Nanosurg 2(1): doi http://dx.doi.org/10.16966/2470-3206.108
Copyright: © 2016 Choudhary S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access