Introduction
In human glomerular disease, chronic proteinuria may lead to proximal tubular damage by several different mediators. Among them, urinary excretion of endothelin-1 (ET-1), originally described as potent vasoconstrictor peptide [1], was found to be correlated with the severity of human renal disease [2-5]. Consequently, several but not all studies with endothelin inhibitors were able to demonstrate a beneficial inhibitory effect by slowing renal disease progression [6-10].
ET-1 mediated signal transduction occurs via an inflammatory signal transduction cascade involving the pleiotropic nuclear factor kappa B (NF-κB) family of dimeric transcription factors [11]. Recently, we have shown that mitogen-activated protein kinase p38α (MAPK p38α) [12], and protein kinase C alpha (PKCα) [13] associate with NF-κB p65 after ET-1 stimulation to form a functional transcription complex (TC). After nuclear transmigration as part of TC, PKCα is able to suppress the release of pri-miRNA15a by direct molecular interaction [13] in resting cells.
MicroRNAs (miRNAs) are short, non-coding RNAs that can play important roles in cell function and development by targeting mRNA sequences of protein-coding transcripts, resulting either in mRNA cleavage or in repression of productive translation in the cytoplasm. In the nucleus, two different forms of an immature miRNA (pre- and pri-miRNA) exist, from which the final, mature, and functional miRNA form is derived [14]. In the case of miRNA15a, ET-1 stimulation leads to a decrease of nuclear PKCα levels. This results in an increase in the production mature miRNA15a, being released in the supernatant [13]. This signaling has been described so far for Caki-1 cells, the malignant variant of proximal tubule cells [13]. Since upregulated levels of miRNA15a have been detected in the urine of renal cancer patients, this miRNA has been suggested as novel marker for clear cell renal cell carcinoma, representing the majority of malignant renal tumors [15].
Knowing that urinary ET-1 levels are upregulated in proteinuric disease, and understanding the regulatory pathway for ET-1 induced miRNA15a release, we hypothesized that this study could yield results in three pathophysiologically relevant, and for diagnostic purposes important issues: i) miRNA15a excretion in the urine as general marker for proteinuria associated with levels of protein or ET-1 or both; ii) miRNA15a as indicator of a glomerular disease helping to differentiate a prolonged, immune complex mediated damage of the glomerular basement membrane (such as in membranous nephropathy) versus a recurrent, transient and “self-healing” disease (such as minimal change nephropathy), and iii) as urinary biomarker for a specific pathway (in this case inflammatory NF-κB), which could be therapeutically targeted, irrespective of the ongoing mediator stimulation leading to proteinuria. The last issue is particularly relevant in familial nephrotic syndromes (i.e. congenital Finnish nephrosis, diffuse mesangial sclerosis, Alport syndrome), in which the underlying genetic alteration leads to chronic proteinuria, and ultimate renal failure, which is rather resistant to treatment. In these cases, detecting the activation of a major inflammatory pathway (via miRNA15a), potentially affecting it by targeting a specific protein (such as PKCα), and in addition being able to monitor the effect of treatment (by reduction of the biomarker miRNA15a in the urine) could be a desirable clinical goal.
Materials and Methods
Materials
Human renal biopsies being formalin-fixed, paraffinized, were used from the archives of the Department of Pathology, University hospital of Koeln, Germany. Five cases each of minimal change, and idiopathic membranous nephropathy as well as five cases of freshly transplanted kidneys (so-called null biopsies) were used (Tables 1a-1e). Urine samples of about 50 to 100 ml were selected from patients and healthy controls according to diagnosis shown in Table 1. 24 hrs urines were collected and samples frozen at -20°C until further use. In selected patients, serum samples of about 1 ml were collected in parallel. Since human materials (serum, renal tissue, urines) were investigated, procedures were followed as outlined in accordance with ethical standards, formulated in the Helsinki Declaration 1975 (and revised in 1983), or the Declaration of Istanbul (for transplant biopsies) respectively, with preapproval by the Ethics Committee at the University Hospital, Koeln (Cologne, Germany; reference number: 09-232). An informed consent from each patient or parents from children was obtained. All reagents were purchased from Sigma-Aldrich (Munich, Germany) unless otherwise specified.
Adult male BALB/c mice were obtained from Charles River (Erkrath, Germany). All animal experimentations have been conducted according to the Guidelines for Care and Use of Laboratory Animals (National Institute of Health, Bethesda, MD, USA), and have been approved by the Ministry of Health (mice: 84-02.04.2012.A118). Every effort was made to minimize the number of animals used and their suffering.
Methods
Animal model of disease: For the Adriamycin model of murine proteinuria, a single tail-vein injection of 10 mg/kg Adriamycin was applied per male BALB/c mouse (20 g body weight; 8 weeks of age; Charles River) [16]. Water and food were allowed ad libitum. Four groups with 10 animals each were investigated receiving the same injection volume in the tail vein: saline or selegiline injected control mice, Adriamycininjected mice, and Adriamycin-injected mice additionally being given an intraperitoneal dose of selegiline in week 3 for 7 continuous days. Selegiline was dosed at 10 mg/kg body weight dissolved in saline [17]. Animals were sacrificed at the end of week 3 by cervical dislocation. Their kidneys were processed by fixation in phosphate-buffered saline and paraffin-embedded. Light microscopic evaluation was performed on H&E sections. 24 hour urine samples, and serum were collected by cardiac puncture.
Cell culture: Primary human renal proximal epithelial tubules cells (RPTEC) were purchased from Pelobiotech (CC-2553) (Planegg, Germany) and cultured according to the manufacturer’s instruction using REBM (Renal epithelial cell base medium, without growth factors, CC-3191) plus the addition of REGM SingleQuots (CC-4127) at 37°C and 5% CO2 .
MDCK cells (Madin-Darby Canine Kidney cells) were purchased from Sigma-Aldrich (84121903-1VL, Munich, Germany) and cultured according to the manufacturer’s instruction using UltraMDCK Serum-free Medium (12-749Q, Lonza, Basel, Switzerland). Passages 6 to 7 cells were employed. For all experiments, cells were pre-incubated for 24 hours with serum-free medium.
The conditionally immortalized human podocyte cell line has been developed by transfection with the temperaturesensitive SV40 T-gene as recently described [18]. Cells were allowed to proliferate at 33°C, and transformed into a quiescent, differentiated phenotype after transfer to 37°C. Culture medium contained standard RPMI supplied with 10% fetal bovine serum (Biochrom, Berlin, Germany) and insulin-transferrinsodium selenite supplement (Gibco, Karlsruhe, Germany). For experiments only cells were used after being cultured at 37°C for at least 14 days until their differentiation was visible by an arborizing morphology.
All cell lines were grown to 80-90% confluence and treated with different mediators for 24 hrs: endothelin-1 (50 nM) [12,13]; transferrin (10 mg/ml) [19]; human albumin (5 mg/ ml) [20]; TNFα (10 ng/ml) [21]; angiotensin (10-7 M) [22]; patient’s urine (1:10 dilution); or freshly isolated complement (diluted 1:10; donated by JWUF) [23]. As controls, RPTEC and MDCK cells were grown without FCS for 24 hrs. Selegiline was used in 1 µmol concentration [24]. The urine sample was filtered (0.2 µm syringe tip filter), a representative sample was routinely analysed by cytologic means using Papanicolaou und Pappenheim stains for cells and signs of infection.
Human biopsies: Formalin-fixed and paraffinized human renal tissue samples from the archives of the Department of Pathology, University Hospital of Koeln, Koeln, Germany were used.
Histologic evaluation was based upon analyses by a staff pathologist (JWUF) using routinely analysed light microscopic (Hematoxilin-Eosin, Periodic acid Schiff, Elastic van Gieson), and immune histologically stained (IgG, IgG4, IgA, IgM, C1q, C3, fibrinogen) paraffin-sections.
Isolation of mouse glomeruli and tubules: Freshly harvested kidneys were cut in coronary sections. Cortex and medulla were separated. Both were independently minced and passaged through graded sieving [25]. The sieves were rinsed several times with PBS and tissue samples collected. Samples were analysed under a cell culture microscope. A 99% pure glomerular fraction was achieved. Samples were frozen at -70°C until further use.
Laser-microdissection of human renal biopsies: Two, 7 µm thick sections each of routinely formalin-fixed, paraffinized, archived human biopsies were cut per case and placed on coated glass slides; 30 glomeruli sections per case were used. After deparaffinisation, glomeruli were microdissected by argon laser and catapulted in a capture device [26]. The remaining cortical tissue was scraped off the slide and used in its entirety. Further preparation was performed using the miRNA isolation kit for FFPE samples from Qiagen. As controls, biopsies from patients being transplanted at time of renal transplantation (“null” biopsies) were used.
miRNA isolation from FFPE samples: For miRNA isolation of the FFPE samples, the miRNeasy FFPE kit from Qiagen was used according to the manufacturer’s protocol [15]. RNA quantification was accomplished using the NanoDrop technology [15].
miRNA isolation from urine: For miRNa isolation from patients’ urine, 1 ml urine was used and added to the Qiazol reagent, mixed, and further used according to the manufacturer’s protocol (miRNeasy Mini kit, Qiagen) [15]. RNA quantification was accomplished using the NanoDrop technology [15].
Quantitative real-time PCR (qRT-PCR): 1 µl of the above mentioned cDNA was used for real-time PCR analysis. The experimental settings were as described previously [12,13]. For quantitative analysis, β-actin was measured. All samples were normalized to β -actin as reference gene. All experiments were done in triplicates. Relative fluorescence was calculated using the ΔΔCT method as outlined in user bulletin 2 (PE Applied Biosystems) [12,13]. The statistical significance of qRTPCR values at different time points was assessed by Student’s unpaired t-test.
The mirVana qRT-PCR Primer Set for miRNA15a was used according manufacturer’s instructions. Primer set for 5s rRNA served to normalize results among different samples [15].
RT-PCR: The cDNA was obtained from 250 ng RNA using random primers and NCode VILO miRNA cDNA Synthesis Kit (Invitrogen) according to the manufacturer’s protocol. The RT-PCR was performed as previously described [12,13].
Statistics
All experiments were performed in triplicates. For the statistical analysis the GraphPrism 5 program was used. Analysis of variance (ANOVA) was performed and the significant differences were calculated by the Newman-Keuls method and indicated by stars (* p<0.05, **p<0.01, ***p<0.001). All differences without indication were not statistically significant.
We analysed body weight and urinary protein excretion values between the two groups of animals injected with Adriamycin or sham operation, before randomizing them for receiving saline or selegeline as treatment, using the two-tailed Student’s t-test.
Reported values otherwise are group means +/- the standard deviation throughout the article. All results involving human urine or tissue analyses (Figures 1-4) were additionally subjected to box blot evaluation to show their degree of dispersion (spread) and skewness in the data.
Results
1. Quantitation of miRNA in urines from adult patients with minimal change (MCN), idiopathic membranous nephropathy (MN) and adult patients with acute renal failure (ARF) due to heart insufficiency
In all 24 hrs urines from adults with minimal change or membranous nephropathy high miRNA15a amounts were detectable by qRT-PCR, ranging between 200 and 600 relative fluorescent units (Figure 1). In contrast, miRNA levels in urines from patients with acute renal failure caused by cardiac prerenal insufficiency were 100 to 300x lower (Figure 1). In pediatric patients high miRNA15a values were detectable in minimalchange nephropathy (Figure 1). Serum levels of miRNA15a were at least 300x lower than urine levels (data not shown).
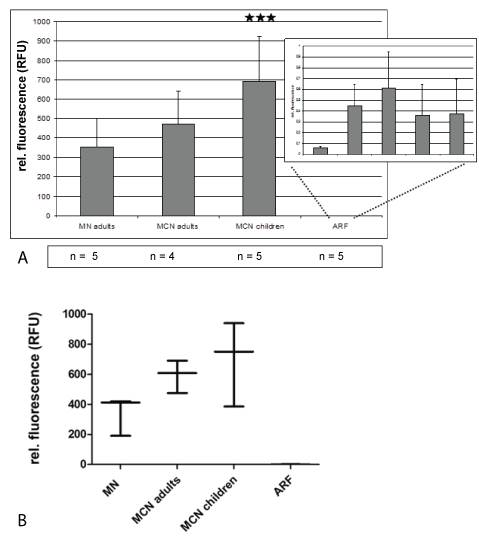
Figure 1: Quantitation of miRNA in urines from adult patients with minimal change (MCN), idiopathic membranous nephropathy (MN) and adult patients with acute renal failure (ARF) due to heart insufficiency
A) In A, mean +/- STD of urines with highly significant expression of urinary miRNA15a in MN and MCD patients. Levels of relative fluorescence vary between 200 and 400 in MCN, and 500 to 700 in MN. Levels in ARF do not reach a relative fluorescence value of 1. Relative fluorescence was calculated using urine from healthy controls (Table 1d); ***p<0.001.
B) In B box plot evaluation of data from A showing the lowest value in protienuric disease for MN is 400 RFUs, for MCD 200 RFUs.
| Patient ID |
Diagnosis |
Age (years) |
Sex |
Creatinine (mg/dl) |
Prot./Crea (mg/dl) |
| 1. GTU 1 |
MN |
75 |
M |
2,07 |
1063 |
| 2. GTU 18 |
MN |
72 |
M |
1,94 |
563 |
| 3. GTU 26 |
MN |
52 |
M |
1,02 |
15614 |
| 4. GTU 32 |
MN |
50 |
M |
1,12 |
489 |
| 5. GTU 45 |
MN |
65 |
M |
2,36 |
2337 |
| 6. GTU 9 |
MCN |
34 |
M |
2,08 |
2566 |
| 7. GTU 5 |
MCN |
35 |
F |
0,89 |
28 |
| 8. GTU 27 |
MCN |
40 |
F |
0,69 |
1350 |
| 9. GTU 17 |
MCN |
62 |
M |
1,60 |
12065 |
Table 1a: Data of adult patients data with glomerulopathies-From simultaneously, routinely performed human renal biopsies tissue slices we used for laser-microdissection analysis.
2. MiRNA15a expression in cultured renal cell lines after stimulation with different mediators for 24 hrs from supernatant versus cellular extracts
A. Human Podocytes
In cell culture the supernatant vs. cell extracts of immortalized human podocytes, stimulated with different mediators, was analysed for miRNA15a (Figure 2A). A 10x diluted urine from a patient with membranous nephropathy, however, showed only a comparatively small miRNA release even if the result is multiplied by its dilution factor 10. ET-1 stimulation also resulted in a relatively small miRNA15a release; all other mediators showed neglectable levels of miRNA15a expression in the supernatant as well as in the cellular extracts. There were no statistically significant differences in the amount of miRNA15a in cell extracts and in the supernatant, except after albumin stimulation: podocytes released a significant amount of miRNA15a in the supernatant, with only minimal amounts remaining in the intact, cultured cells as detected by qRT-PCR.
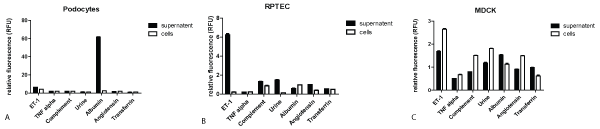
Figure 2: MiRNA15a expression in cultured renal cell lines after stimulation with different mediators for 24 hrs from supernatant versus cellular extracts-All cell lines were stimulated for 24 hrs with reagents shown; concentrations were selected according to the ones established in the literature (see method section). Supernatant (black columns) and cell extracts (white columns) were collected and analysed for miRNA15a by qRT-PCR. * p<0.001.
A) Human podocytes: maximal release for miRNA15a in supernatant after albumin stimulation.
B) Human RPTECs: ET-1 leads to maximal release of miRNA5a in the supernatant.
C) MDCK cells: miRNA15a is detectable only in small amounts with slightly higher values in the cell extracts than in the supernatant (except for transferrin).
B. Human RPTECs
In cell culture the supernatant vs. cell extracts of RPTECs, stimulated with different mediators, was analysed for miRNA15a (Figure 2B). ET-1 stimulated most effectively with more than 90% of all miRNA15a made being released. The effect of other mediators is less than 10% of that of ET-1 both, in the supernatant and in the cellular extracts, particularly including albumin. In contrast to human podocytes, in a patient with membranous nephropathy the urine plays a more important role in stimulating miRNA15a release. Accounting the dilution factor, the final result would be about 66% higher than the stimulation with ET-1 alone, so that it seems likely that several different mediators acting together leading to this result besides albumin alone.
C. MDCK cells
In cell culture, the supernatant vs. cell extracts of MDCKs, stimulated with different mediators, was analysed for miRNA15a (Figure 2C). Overall level for all mediators were 1.5 RFUs or less in both, cell line and supernatant except after ET-1 stimulation. Here in contrast to RPTECs, the amount of miRNA15a was significantly lower with a reverse relationship between cells and supernatant, the latter being lower in value (except for transferrin).
3. Experimental therapeutic intervention using selegeline
A. MiRNA15a expression in the supernatant of cultured human RPTECs
Since selegiline induces PKCα, RPTECs were pre-treated with selegiline following ET-1 stimulation. MiRNA15 levels were drastically reduced in the supernatant being lower by about a factor 350x compared to ET-1stimulated cells (Figure 3A). Using albumin as stimulator, selegiline treatment reduced the induction of miRNA15a expression in the supernatant by about 50%.
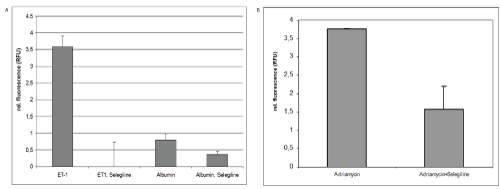
Figure 3: Experimental therapeutic intervention using selegeline
A) MiRNA15a expression in the supernate of cultured human RPTECs-After 24 hrs treatment with ET-1, human proximal tubule cells show an increased amount of miRNA15a in the supernatant versus untreated controls. This miRNA induction can be completely reversed by simultaneous treatment with selegiline; ***p<0.001. After 24 hrs treatment with human albumin, a small amount of miRNA5a can be detected in the supernatant, which is reduced by selegiline treatment to 50%; * p<0.5.
B) Results of urinary miRNA 15a expression in Adriamycin induced proteinuria-A single tail-vein injection of Adriamycin induced the classic nephropathy reported in the literature [16] with a glomerular damage leading to a proteinuria of an average of 180 mg/d by week 3. Urine qRT-PCR analysis of miRNA15a levels were significantly increased over saline injected control animals (as calculated by rel. fluorescence). Animals being treated with selegiline (10 mg/kg body weight for seven consecutive days) in the third week after Adriamycin injection only reached relative fluorescence values of 1.8 on average, while untreated Adriamycin animals have relative fluorescence levels of 3.8 compared to controls; * p<0.05 versus Adriamycin treatment alone.
B. Results of urinary miRNA15a expression in Adriamycin induced proteinuria
Adriamycin treated mice were proteinuric (180+23 mg) at end of week 3, irrespective of treatment. Control animals (saline) showed no detectable proteinuria. In Adriamycin treated animals, urinary levels of miRNA15a reached up to 4 relative fluorescence units (Figure 3B, left column). No increase in miRNA15a was detectable in the serum of Adriamycin mice (data not shown).
Morphologically, Adriamycin treated animals showed individually dilated tubules with protein casts. Increased protein reabsorption was present (data not shown).
Adriamycin treated mice were randomly selected for selegiline treatment with matching levels of proteinuria between selegiline/Adriamycin treated and Adriamycin-only treated group. Solely selegiline treated control animals were not proteinuric nor did they display any histologic damage (data not shown). Following selegiline treatment, levels of miRNA15a were reduced by 60% (Figure 3B, right column) compared to exclusively Adriamycin treated animals (Figure 3B, left column). However, the level of proteinuria was not affected by selegiline treatment (data not shown). Using miRNA15a levels in the urine as indicator of the activity of the inflammatory NF-κB pathway in proximal tubules/podocytes, the reduction of its levels by selegiline indicates, that this pathway can be downregulated in these cells, while typical mediators such as proteinuria and ET-1 are still present. Thus, if proteinuria cannot be completely prevented, its effect on proximal tubules/ podocytes as proinflammatory mediator via NF-κB may still be blocked as indicated by downregulated levels of miRNA15a.
4. Laser-microdissection analysis for quantitation of miRNA using formalin-fixed, paraffinized glomeruli and cortical tubular fractions
A. Adult patients with idiopathic MN versus control cases from routine transplant biopsies
For laser-microdissection human renal biopsies from membranous nephropathy, minimal change disease (Table 1a and 1b) or controls (“null” biopsies, Table 1e) were used. Ten glomerular slices were microdissected. From the obtained cortical tissue miRNA was extracted separately and analyzed by qRT-PCR. The tubular fraction showed significantly higher values for miRNA than the glomerular fraction (Figure 4A).
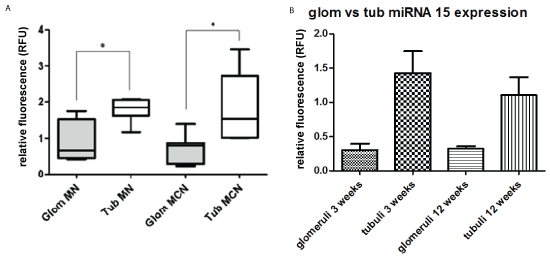
Figure 4: Laser-microdissection analysis for quantitation of miRNA using formalin-fixed, paraffinized glomeruli and cortical tubular fractions
A) Adult patients with idiopathic MN versus control cases from routine transplant biopsies-For laser-microdissection human renal biopsies from membranous nephropathy, and minimal change disease (Tables 1a and 1b) or controls (“null” biopsies, Table 1e) were used. Ten glomerular slices were microdissected. From the obtained cortical tissue miRNA was extracted separately and analyzed by qRT-PCT. The tubular fraction showed significantly higher values for miRNA than the glomerular fraction (Figure 4A). Control cases used are listed in Table 1e. MN membranous nephropathy; MCH minimal change disease; * p<0.05.
B) Mice with Adriamycin nephropathy-MiRNA15a expression was analysed in glomeruli vs. tubules after graded sieving 3 vs. 12 weeks after a single dose of Adriamycin. At both time points, the amount of miRNA15a was significantly higher in proximal tubules than in Lasermicrodissected glomeruli; * p˂0.05.
| Patient # |
Diagnosis |
Age (years) |
Sex |
Proteinuria (g/24hrs) |
| 1. |
MCN |
6 |
M |
5 |
| 2. |
MCN |
4 |
M |
3,64 |
| 3. |
MCN |
14 |
F |
4 |
| 4. |
MCN |
3 |
M |
11,16 |
| 5. |
MCN |
9 |
M |
8,5 |
Table 1b: Data from pediatric patients with minimal change nephropathy (MCN)-In each patient, creatinine (lower 0.9 mg/dl) and urea (lower than 45 mg/dl) levels were within normal limits.
| Patient ID |
Diagnosis |
Age (years) |
Sex |
Creatinine (mg/dl) |
Oliguria (ml/hr) |
AKIN
Stage |
| 1 |
Bypass Op, HD |
78 |
F |
-- |
20 |
1 |
| 2 |
Heart insuff., HD |
55 |
M |
1,0 |
-- |
3 |
| 3 |
NSTEMI,
Septicemia |
78 |
M |
0,8 |
? |
2 |
| 4 |
ARF, prerenal |
74 |
M |
0,7 |
20 |
1 |
| 5 |
ARF, prerenal |
57 |
M |
0,9 |
20 |
1 |
| 6 |
HD |
89 |
M |
1,0 |
20 |
3 |
| 7 |
CHD, ACVB |
73 |
M |
0,9 |
20 |
2 |
| 8 |
CI, brain death |
42 |
M |
1,1 |
30 |
3 |
Table 1c: Adult patients with acute renal failure-Patients were classified according to the acute renal injury (AKIN) stadium [27]; HD: Hemodialysis; ARF: Acute renal failure; CHD: Chronic heart disease; ACVB: Acute coronary venous bypass; CI: Cardiac insufficiency.
| Control ID |
Age (years) |
Sex |
| MN |
24 |
F |
| NE |
28 |
F |
| MK |
30 |
M |
| SK |
29 |
M |
| LE |
64 |
M |
| VD |
66 |
M |
Table 1d: Adults as healthy controls.
| Patient # |
Diagnosis |
Age (years) |
Sex |
| 1 |
NTX |
44 |
M |
| 2 |
NTX |
68 |
M |
| 3 |
NTX |
36 |
M |
| 4 |
NTX |
39 |
M |
| 5 |
NTX |
60 |
M |
Table 1e: Data from patients with “null” biopsies serving as control renal tissues for laser-microdissection.
B. Mice with Adriamycin nephropathy
Using renal cortical slices for graded sieving, glomeruli were separated from tubules. By qRT-PCR, the level of miRNA15a found in proximal tubules was about 4x higher than in glomeruli at 3 weeks, and about 3x higher at 12 weeks after Adriamycin injection (Figure 4B). Glomerular and tubular fractions from untreated control littermates were used to calculate relative fluorescence.
Discussion
We started this investigation to analyse the diagnostic role of the inflammatory NF-κB pathway using miRNA15a as mechanism-specific marker in proteinuric disease. From the results found, we postulate that miRNA15a is: i) principally upregulated in proteinuria, which is important to monitor its reduction after targeted therapy; ii) it is also activated by ET-1 as one of the major mediators in proteinuric disease. However, its detection in the urine in proteinuric patients does not allow to differentiate well-known glomerular diseases associated with proteinuria (i.e. MN vs. MCN). Furthermore, since the activation of the inflammatory pathway takes place in podocytes and proximal tubules alike, analysing urine miRNa15a levels does not allow to determine the individual contribution of different cell types.
In contrast, miRNA15a can be a specific urinary biomarker for the inflammatory NF-κB pathway to monitor the effect of targeting PKCα, irrespective of the ongoing mediator stimulation leading to proteinuria. Thus, it could be a novel diagnostic tool for detection, and treatment monitoring of the activity of the NF-κB inflammatory signaling in chronic proteinuric diseases such as the familial nephrotic syndromes (i.e. congenital Finnish nephrosis, diffuse mesangial sclerosis, Alport syndrome), where the underlying genetic alteration causes problems in effective treatment. For this purpose it was crucial to pre-characterize the underlying signaling cascade [12,13].
We propose a novel strategy of using miRNAs as biomarker for damage of proximal tubules caused by glomerular disease with proteinuria and increased urinary Endothelin-1 levels. Previously, we have shown that this concept is successful in the mechanism of proximal tubular cytotoxicity [27]. We found that ET- via its Endothelin-B- receptor is able to upregulate miRNA133a in proximal tubules. By interaction with the 3’UTR domain of the multiple-drug associated protein 2 (mrp2), miRNA133a leads to downregulation of this transporter complex. This mechanism was detectable in human diseases such as MN and MCN as well as in the mouse Adriamycin model, in which high urinary levels of miRNA133a were associated with low levels of mrp-2 Protein.
To postulate miRNA15a as urinary biomarker for a disease mechanism we tested whether this concept was (i) true in proximal tubular epithelium; (ii) a useful proof of principle for therapeutic protection for ET-1 as proteinuric mediator.
To begin with, a reliable urinary biomarker was needed, which is only detectable in patients with chronic proteinuric disease but in no other renal diseases. In proteinuric membranous or minimal change nephropathy, miRNA15a can be easily detected in high relative fluorescent units by qRTPCR (Figure 1). In contrast, diseases such as acute renal failure, causing destruction of epithelial integrity and being expected to release their miRNAs, only showed insignificant levels of urinary miRNA15a. This should make miRNA15a an ideal marker in routine clinical practice.
MiRNA15a is also suited since it is detectable in the same disease entities in adults and in children (Figure 1). Its reliable detectability in patients’ urine even after several freeze-thaw cycles makes it extremely useful (data not shown). The model of Adriamycin toxicity leading to proteinuria is a suitable study model, since miRNA15a was highly expressed in the urine of proteinuric mice (Figure 3B).
Secondly, the marker should be as specific as possible for damage of the cellular target. Laser-microdissection of human renal biopsies of MN or MCD versus controls from biopsies of freshly transplanted kidneys – so-called null biopsies - showed that miRNA15a was most prevalent in proximal tubules, and to a smaller extent in glomeruli (Figure 4A). This result was supported in the mouse model by graded sieving. The tubular fraction contained the majority of miRNA15a at 3 weeks (Figure 4B). However, since there is currently no individual protein known in the NF-κB signaling pathway leading to miRNA15a upregulation, which is specific for this pathway only either in podcytes or in proximal tubules, the levels of miRNA15a measured in the urine can be derived from different cell sources along the nephron such as podocytes as well as proximal tubule cells.
The detectability of this miRNA for the entire duration of the disease process is important, since in human disease the exact starting point of the disease is often unknown. In Adriamycin nephropathy, increased miRNA15a levels could be detected predominantly in proximal tubules up to a 12 week period (Figure 4B). In contrast, in non-proteinuric human MCD, miRNA15a was no longer detectable (unpublished data).
Thirdly, since different mediators can be found in the urine in proteinuric disease [5-7,20-23], a cell culture experiment was performed using immortalized human podocytes, human RPTECs, and MDCK cells (Figure 2). MiRNA15a was mainly upregulated by albumin in podocytes (Figure 2A) and by ET-1 in proximal tubules (Figure 2B), and in comparison insignificantly by other mediators. This indicates that at least within this in vitro system for miRNA15a activation, complement, transferrin or angiotensin do not play any significant role in contrast to ET-1. MiRNA15a levels after ET-1 stimulation in cultured proximal tubules could only be matched by the results from a patient’s urine (MN; 10 g proteinuria/d) by multiplying with the 10x dilution factor (Figure 2b). Human serum albumin stimulation did not achieve levels comparable to those of ET-1 in proximal tubules, while albumin was a major mediator for immortalized podocytes.
The discrepancy between the miRNA15a levels in vitro versus in vivo may have different reasons: pathophysiologic mechanisms (immunologic vs. non-immunologic); other mediators; higher cell number in the patient participating in the miRNA release compared to in vitro conditions. Even if the absolute contribution of other mediators to the miRNA levels is much smaller than that of ET-1 for proximal tubule cells, the amount measured in the urine is the entire amount of miRNA collected from all possible sources. Thus, one should not expect similar levels between cell culture and patients. In humans, proximal tubules seem to release the majority of the generated miRNA in the urine, leading to small levels detectable after Laser-microdissection. Finally, other cells may be suspected to have contributed to the urinary miRNA levels, such as collecting duct cells. However, while these cells in vitro could be stimulated principally by the applied mediators used (Figure 2C), its clinical importance is uncertain since this is a crossspecies comparison (human RPTECs vs. dog MDCK cells).
To confirm the renal origin of the miRNA, we compared the miRNA results directly between urine and serum from the same proteinuric patients. We could only detect high miRNA levels in the urine; blood levels were at least 300x lower. This result is in good agreement with significantly higher levels of miRNA15a in the urine of clear cell carcinoma, originating from the proximal tubules, compared to the serum of the same patient [15]. All these investigations indicate that proximal tubules (and to a lesser degree the glomeruli) are the major contributors to miRNA15a measured in the urine in proteinuric disease.
Finally, we tested whether this miRNA being a potential biomarker for downregulation of a signaling pathway (PKCα), may also be an indicator for a successful therapy. Applying selegiline in vitro, an upregulator of PKCα levels in neural cells [28], also drastically reduced miRNA15a levels in ET-1 stimulated RPTECs to non-stimulated, background values (Figure 3A). In the Adriamycin model, high levels of urinary miRNA15a could be reduced by 50% after treatment with selegiline in the last week of a 3 week experimental period (Figure 3B). Thus, this therapy is well tolerated and effective even after a disease process has begun and its effect is detectable by measuring urinary miRNA. Since this medication is used in human therapy already [29], monoamine oxidase inhibitors may potentially provide a new therapeutic approach to upregulate PKC levels in human proteinuric disease to prevent the activation of an ET-1 mediated inflammatory signaling cascade. However, just as miRNA15a is not entirely specific for one individual cell type along the nephron only, but originates from podocytes and proximal tubule cells as shown by cell culture experiments (Figure 2), the observed effect of selegeline is an overall net result on miRNA15a from this pathway in both cell types.
The problem of chronic proteinuria is the development of proximal tubule epithelial-mesenchymal transition (EMT), and interstitial fibrosis [30,31] via increased amounts of NF-κB in proteinuric animal models. Attempts to prevent this detrimental process need the use of an efficacious medication to control the NF-κB increase and consequently reduce EMT and interstitial fibrosis. For the first requirement, we showed that selegiline is such a medication, since - in a murine Adriamycin model of proteinuria - it prevented the maturation of miRNA15a which is otherwise responsible for the degradation of a cytoplasmic transcription complex, allowing increased nuclear migration of NF-κB. Thus, urinary levels of miRNA15a are an efficient indicator for a successful therapy to block the inflammatory NF-κB pathway.
For the second requirement, one would have to show whether blocking the NF-κB pathway alone or in combination with others is sufficient to prevent EMT, and chronic renal failure. This needs future studies with different disease models, requiring a prolonged treatment period, in which differences between treated and untreated animals may become visible, such as a proteinuric rat model [32]. This treatment concept is similar to that in oncology, where for instance a mutated receptor such as EGFR leads to a constitutive activation of a pathway being blocked by downstream target such as tyrosine kinase inhibition. In our case, the “constitutive activation” of the inflammatory pathway is provided by chronic proteinuria, which cannot be sufficiently prevented or blocked. Selecting a “downstream target”, here NF-κB, to inhibit therapeutically by selegiline, might prevent a certain negative result (namely EMT, and interstitial fibrosis: to be shown), although the mechanism itself (namely proteinuria) is ongoing.
The lack of apparently effective medications to protect proximal tubule cells from the harmful effects of mediators released under proteinuric conditions such as ET-1 is in part due to the inability to control, whether applied medications actually reach the proximal tubule cells in sufficient concentration. This could be achieved using biomarkers such as miRNA15a, which would monitor the effects of these medications; i.e. if the activation of the signaling loop is stopped, miRNA15a levels should fall or be no longer detectable in the urine.
In summary, while these experiments have to be substantiated further, one may discern that miRNAs such as miRNA15a may indeed be useful as biomarkers for diagnosis and for monitoring therapeutic efficacy of specific pathways in proximal tubular cells under proteinuric conditions, particularly when mediated by ET-1. Furthermore, our study may be also an encouragement for investigations of this concept, particularly when genetically based diseases such as in congenital nephrotic syndromes, primary FSGS or tumor-induced MN do not allow to overcome the essential pathologic mechanism of chronic proteinuria.
Funding Sources
Heike Loeser is supported by the Koeln Fortune Program/ Faculty of Medicine, University of Cologne. Melanie von Brandenstein has a postdoctoral fellowship from Koeln Fortune Programm/University of Cologne. The work was supported by a grant from the Marga und Walter Boll Stiftung (to J.W.U.F).
Conflict of Interest
None.
Acknowledgements
The corresponding author (J.W.U.F) is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. For assistance with medical English we are indebted to Mrs. Anne Schofield-Fries.





