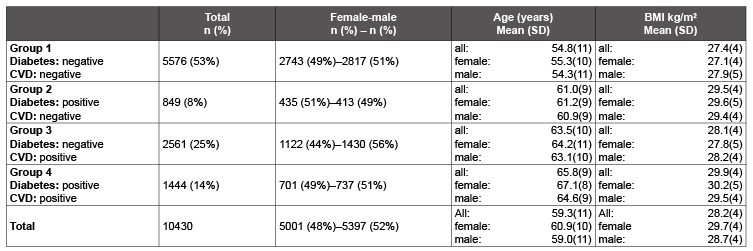
Table 1: Demographic data at the beginning of the study

Hostalek U1* Czarnecka D2 Kurzeja A3 Koch EMW4
1Merck KGaA, Darmstadt, Germany*Corresponding author: Hostalek U, Merck KGaA, Darmstadt, Germany, Tel: 01511 454 3106; E-mail: ulrike.gottwald-hostalek@merckgroup.com
Study objective: The data from a non-interventional study in more than 10,000 patients were assessed to analyse relationships between hypertension and diabetes or cardiovascular diseases. A further aim was to investigate the effect exerted by treatment with a fixed-dose combination (FDC) of bisoprolol and amlodipine in respect of patient adherence and blood pressure control.
Material and methods: The data from the baseline findings of patients receiving treatment with bisoprolol and amlodipine were recorded in case report forms and compared with the data from the findings following 6months of treatment with the corresponding FDC. Only minor changes to the original daily dose of both active substances were made for the switch. Four groups were formed: 1. Without comorbidities, 2. diabetespositive, 3. cardiovascular disease-positive, 4. diabetes- and cardiovascular disease-positive.
Type 2 diabetes mellitus was diagnosed following the American Diabetes Association (ADA) criteria: fasting plasma glucose (FPG) >125 mg/ dl (>6.9 mmol/l), and/or a 2-h plasma glucose (2-h PG) value after a 75-g oral glucose tolerance test (OGTT) >199 mg/dl (>11.0 mmol/l) and/or an HbA1C >6.4%.
Adherence was determined on the basis of the consumption of the prescribed tablets: excellent >90%, good: 76-90%, moderate: 51-75%, poor ≤ 50%. The effect was assessed on the basis of the reduction in blood pressure and pulse pressure values and by calculating the proportion of patients with a target blood pressure value <140/90 mmHg.
Results: The data from a total of 10,430 patients were used for the comparative evaluations. Group 1 included 53% of the patients at baseline with a mean blood pressure of 145.5/88.3 mmHg. The proportion of patients >140/90 mmHg was 28%. The following data were obtained for the groups with comorbidities:
Group 2 (8%): mean blood pressure: 149.3/89.2; 33% >140/90 mmHg
Group 3 (25%): mean blood pressure: 148.5/88.3; 31% >140/90 mmHg
Group 4 (14%): mean blood pressure: 151.5/90.1; 38% >140/90 mmHg.
The mean dose of the fixed-dose combination in group 1 was 5.7 ± 2 mg/day for bisoprolol and 6.3 ± 2 mg/day for amlodipine. The highest dose was prescribed to patients in group 4:6.0 ± 2/6.6 ± 2 mg/day.
After 6 months, good to excellent adherence was recorded for 95% of patients in all groups. The mean systolic blood pressure values were: Group 1: 130 ± 9, Group 2: 131.7 ± 11, Group 3: 131.3 ± 10, Group 4: 132.3 ± 10 mmHg. Pulse pressure decreased similarly with an effect strength d >0.5 in all groups.
Conclusion: The switch from a free to a fixed-dose combination of the antihypertensive agents bisoprolol and amlodipine results in good to very good adherence in patients. This is accompanied by a significant reduction in previously increased blood pressure values in most cases to below the target value of 140/90 mmHg, irrespective of any existing comorbidities such as diabetes and cardiovascular diseases, indicating a reduction in the risk of fatal events.
Hypertension; Blood pressure reduction; Fixed-dose combination; Comorbidities
Patients with hypertension represent the most frequent cause of medical management to reduce the life-threatening risk associated with myocardial infarction, stroke or renal failure [1]. According to recent evidence based findings, subjects over 60 years of age should have a target value of less than 150/90 mmHg. The generally recommended aim is to achieve blood pressure values of less than 140/90 mmHg, in the process of which medications must be individually adjusted and the occurrence of adverse reactions or impairment of quality of life due to these prescribed medications should be avoided. The same recommendations have also been published for patients over 18 years of age with diabetes mellitus.
If it is not possible to achieve the targeted blood pressure values with the prescription of an antihypertensive medication, a combination of substances with different mechanisms of action should be prescribed.
Guidelines were presented at the ESC Congress 2013 that required the individualisation of therapy in patients with diabetes, cardiovascular diseases and hypertension [2-4]. Risk stratification was recommended in order to select suitable therapeutic agents. The new guideline on hypertension also specifies a standard limit or target value of 140/90 mmHg (self-measurement by the patient: 135/85 mmHg). These values should also be an aim for multimorbid patients, but in all cases the mean 24-hour value should not exceed 130/80 mmHg.
A further reduction, particularly of diastolic blood pressure, may be associated with an increased risk for myocardial infarction in patients with coronary heart disease (CVD) [5]. This is the so-called J-curve phenomenon [6].
The main antihypertensive product classes, such as diuretics, angiotensinconverting enzyme (ACE) inhibitors, calcium channel blockers or betablockers, exhibit a more or less similar hypotensive effect [7].
Beta-blockers, such as bisoprolol, have been prescribed for several decades for the treatment of cardiovascular diseases [8-11]. As the results of the CIBIS studies confirm, the selective effect on the beta-1 adrenergic receptor favours bisoprolol, especially for patients with heart failure [12].
The combination of bisoprolol with a calcium channel blocker such as amlodipine has proved effective for the treatment of many patients with high blood pressure values [13-16].
In order achieve the absolutely essential adherence required for such a combination, tablets containing the most widely prescribed dosages in a fixed formulation have been developed (5+5, 5+10, 10+5 and 10+10 mg bisoprolol and amlodipine).
The data from a non-interventional, multicentre cohort study in more than 10,000 patients [17] confirmed that these fixed-dose combinations (FDC) yielded good to excellent adherence in more than 95% of patients over the 6 month study period. This outcome was associated with a significant reduction in blood pressure values, with pulse pressure falling by an average of 11%.
By virtue of their large case numbers and the non-selective choice of patients under everyday clinical practice conditions, such observational studies offer the possibility of analysing further aspects in relation to additional factors in order to obtain further findings for optimising diagnosis and therapy.
On the basis of the data from the previously mentioned multicentre study, the following aspects were evaluated:
What proportions of patients with raised blood pressure also suffer from diabetes and/or coronary heart diseases (CVD).
What differences in the blood pressure profile are seen in patients with diabetes/coronary heart diseases and hypertension compared with patients without these comorbidities.
What dosage regimens are prescribed for hypertensive patients with and without comorbidities.
What differences there are in dosage regimens between free and fixeddose combinations.
What differences there are in the adherence of patients with and without comorbidities.
What changes in blood pressure values are seen after a 6-month treatment with a fixed-dose combination (FDC) of bisoprolol and amlodipine.
What differences exist in the blood pressure profile after 6 months of use of the FDC.
In the context of this non-interventional study, hypertensive patients aged over 18 years who had been switched from a free combination of bisoprolol and amlodipine to the FDC at least 4 weeks before recruitment were selected.
No additional treatment measures that differed from the clinically appropriate management of the patients were prescribed. Any additional medications or non-pharmacological treatment measures required were used in accordance with medical instructions; co-administration of any other antihypertensives was considered an exclusion criterion.
Blood pressure measurements were taken in the upright seated position after a 5-minute rest period. Patients were given a prescription for a specified number of tablets for the treatment period up to the next follow-up visit (mandatory after 6 months) and their consumption was checked. The recording of findings from the screening examinations was repeated after 6 months and the results documented. The entries in the case report forms were transferred to the BIAS (Biometric Analysis of Samples, Hanns Ackermann, Frankfurt) analysis program and used for comparative analyses.
Using this program, comparative calculations were made of the means, standard deviations, confidence intervals, medians and quartiles. Contingency tables were formed for comparison of the data and analysed by the Mantel-Haenzsel test. Effect strengths (Cohen’s D) were determined to compare the variables. In view of the large number of cases, no statistical calculations of p values were performed.
For the comparative study, 10,430 patients for whom data were available at the beginning of the study on the diagnoses of “diabetes mellitus type 2” and “concurrent cardiovascular disease (CVD)”were selected (Table 1). In 53% of patients neither diabetes nor CVD was present. There were no relevant differences between the four groups in respect of age, sex distribution and body mass index (BMI).
In principle, the mean measurements show that hypertension as defined by the WHO was present in all 4 groups despite free-dose combination therapy (mean duration 1.5 (SD ± 22) months) with bisoprolol and amlodipine.
In terms of the target values of 140/90 mmHg, there were marked differences between groups 1 to 4.
The number and proportion of patients with blood pressure values greater than 140/90 mmHg were as follows: in Group 1: 1537 (28%); in Group 2: 280 (33%); in Group 3: 794 (31%); and in Group 4: 551 (38%).
Between-group comparisons reveal that the occurrence of both comorbidities in particular is associated with a confirmed higher percentage of patients with uncontrolled blood pressure. In patients without these comorbidities, the percentage of uncontrolled blood pressure patients is much less. Table 2 compares the baseline blood pressure values for all 4 groups.
Table 3 is presents the distribution of systolic blood pressure and pulse pressure for all patients at study start. In the case of systolic blood pressure, there are marked differences between groups 1 and 3, 1 and 4, and 2 and 4. In the case of pulse pressure, there is a confirmed difference between group 1 and the other groups.
Table 4 compares the mean doses per day for bisoprolol and amlodipine before the beginning of the study as free combinations and after the switch to the fixed-dose combinations. On average relatively small increases were made in the daily dosage of the individual substances. The switches occurred on average after 5.5 weeks.

Table 1: Demographic data at the beginning of the study
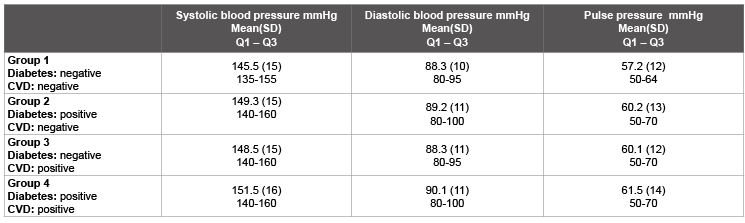
Table 2: Blood pressure values at the beginning of the study
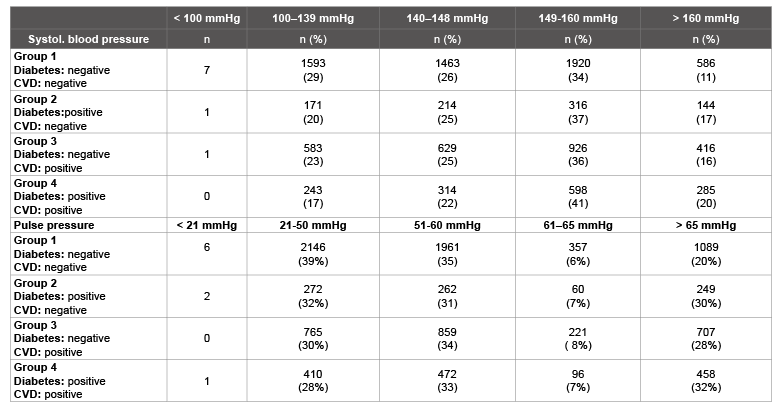
Table 3: Distribution of systolic blood pressure and pulse pressure at study start
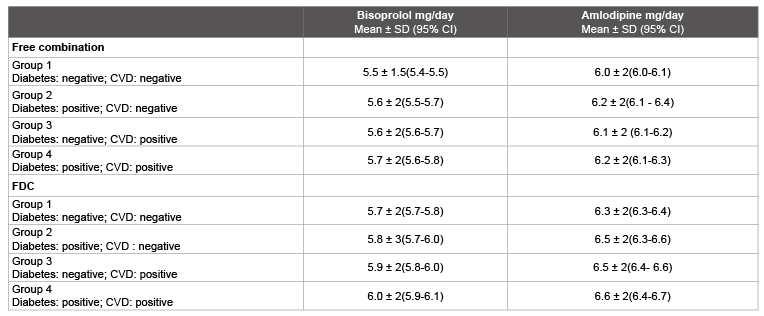
Table 4: Comparison of the mean daily doses for bisoprolol and amlodipine as a free combination and after switching to the FDC
In the comparisons of the percentage distribution of patients between the individual dosage regimens (Table 5), it is striking that a marked increase in dose was undertaken in all groups in connection with the switch from free to fixed dose combinations of the two antihypertensives.
There are also indications that between the individual groups the dosages were lower for patients without comorbidities.
After a six-month treatment period, excellent adherence was found in patients in all groups without detectable differences (Table 6).
Table 7 compares the blood pressure values after 6 months of participation in the study. The follow-ups after 6 months revealed clinically relevant reductions in mean systolic blood pressures by more than 10% in all groups, which resulted in high values in the effect strengths. The mean differences in systolic blood pressure values from the baseline values were as follows: in Group 1: 12.7 ± 14 mmHg: effect strength d: 0.9; Group 2: 17.7 ± 17 mmHg; effect strength d: 1.1; Group 3: 17.1 ± 16 mmHg; effect strength d: 1.1; and Group 4: 19.3 ± 17 mmHg; effect strength d:1.1.
The higher baseline values for groups 2, 3 and 4 resulted in a somewhat more marked decrease in systolic blood pressure in these patients. The confirmed reduction in the proportion of patients with blood pressure values above the target value of 140/90 mmHg in all 4 groups is particularly striking, so that no further differences were observed:
Group 1: n=43 (1%), Group 2: n=7 (1%), Group 3: n=35 (2%), Group 4: n=5(2%).
Some differences between the groups were also seen in the differences in pulse pressure values, which can be ascribed to the baseline value concerned:
Group 1: 6.2 ± 13mmHg; d: 0.54, Group 2: 7.8 ± 14 mmHg: d: 0.55, Group 3: 8.0 ± 14 mmHg; d: 0.63, and Group 4: 8.5 ± 15 mmHg; d: 0.58.
Table 8 presents the distribution of systolic blood pressure and pulse pressure after 6 months of treatment. Markedly positive changes were observed in all groups in terms of the proportion of blood pressure values within the normal range (≤ 140/90 mmHg).
The differences between the individual groups are minor.
The heart rates for the patients also changed in all 4 groups during the 6-month treatment with the FDC:
Group 1: baseline value: 75.5 ± 10; final value: 68.6 ± 7 bpm
Group 2: baseline value: 76.7 ± 10; final value: 68.7 ± 7 bpm
Group 3: baseline value: 76.2 ± 10; final value: 68.4 ± 7 bpm
Group 4: baseline value: 77.2 ± 10; final value: 68.9 ± 7 bpm.
At the end of the study, all patients where asked whether they would prefer the free or the fixed dose combination. About 90% of patients in all groups answered that they would prefer the FDC. Overall, the FDC of bisoprolol and amlodipine was well tolerated. Totally, 89 adverse events were reported in 70 patients (0.7%). The majority of the adverse events were edema (41 AEs in 41 patients, 46.1%), followed by headache (7 AEs in 7 patients, 7.8%), dizziness (6 AEs in 6 patients, 6.7%), and bradycardia, nausea and skin burning/redness (4, 4.5% each). Only 3 adverse events (3.4%) were considered serious, one case of atrial fibrillation (not related), one case of chronic heart failure worsening, and one head injury leading to death (not related). Totally, only 9 patients out of 10,532 patients (0.09%) discontinued the study due to adverse events.
Non-interventional studies (NIS) are defined as studies in which findings from the treatment of people with medicinal products are analysed. In the process, treatments, including diagnosis and monitoring, are undertaken not on the basis of a pre-established study plan, but in medical practice in accordance with the requirements defined in the authorisation for the use of the therapeutic agent [18,19]. With good organisation and monitoring, consistent results are obtained from these studies with a low probability of error and sufficient power to prevent incorrect conclusions [20,21].
The results of this analysis are based on the data from a noninterventional observational study conducted in two stages, in which the use of the fixed-dose combination (FDC) of bisoprolol and amlodipine in more than 4000 patients in the first part [22] resulted in the same good adherence and blood pressure reduction as in the follow-up study with more than 6000 additional patients [17]. Others have noted that single-pill regimens are associated with a more rapid achievement of blood pressure control and a better adherence to therapy [23,24], and are highly costeffective [25].
The extensive data from a total of more than 10,000 patients allowed a further analysis focusing particularly on patients diagnosed with diabetes and/or a concurrent cardiovascular disease (CVD) in addition to hypertension.
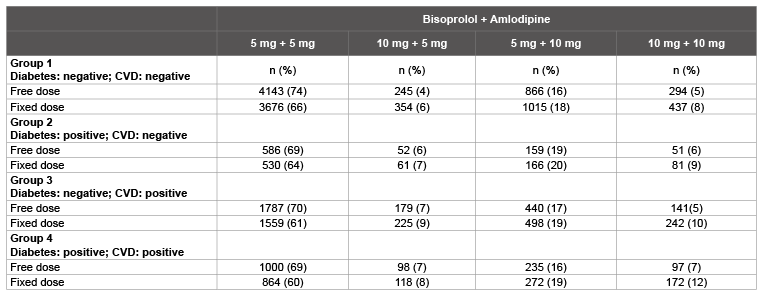
Table 5: Mean dose of bisoprolol and amlodipine in the groups
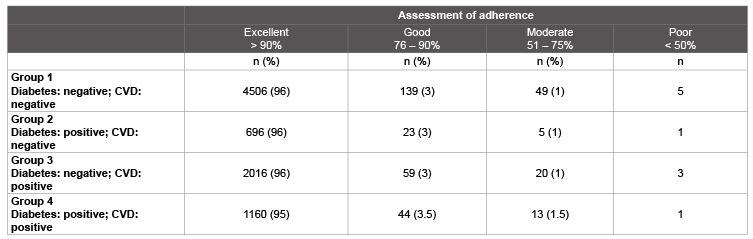
Table 6: Patient adherence after 6 months
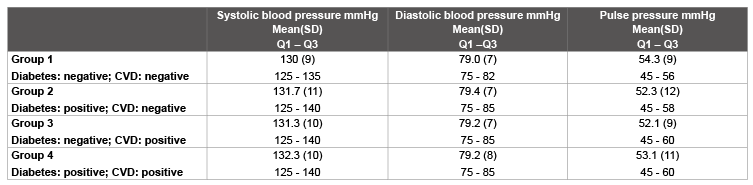
Table 7: Comparisons of blood pressure values after 6 months
The proportion of these patients assigned to 3 reference groups was 47%, with 14% having to be assigned to both diagnoses. As a result, considerable similarities were seen with the results of epidemiological studies [26].
Diabetes is generally described as a high-risk condition because of its close association with cardiovascular diseases. The situation is often particularly complex because of coexistent neuropathic and microangiopathic disorders.
The comparative study results show that patients with diabetes and CVD are on average older than patients diagnosed with hypertension alone. The higher BMI figures in patients with diabetes are also significant.
The increased risk from comorbidities was also seen in the markedly increased proportion of patients with blood pressure values greater than 140/90 mmHg. For the overall population, a median systolic blood pressure of 140 mmHg was calculated before the beginning of the study and 45% of study participants without comorbidities had a higher value. In patients with diabetes/CVD, the proportion was between 50 and 60%. The profile of these data is similar to that for the pulse pressure values in the individual groups.
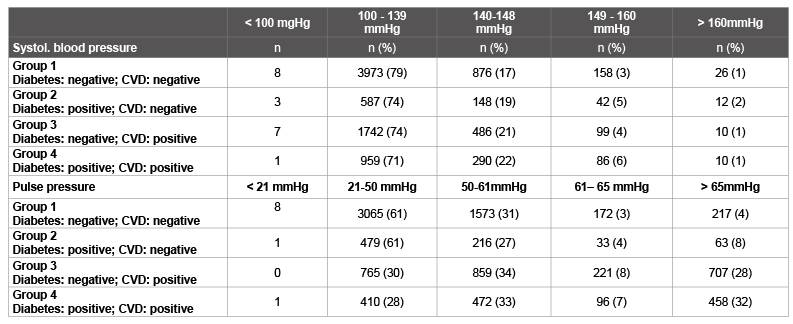
Table 8: Distribution of systolic blood pressure and pulse pressure after 6 months of treatment
The treating doctors had attempted to control blood pressure values, to a large extent without sufficient success, by the combined use of the two antihypertensives, which involved the prescription of somewhat higher mean doses in view of the higher risk for patients with diabetes/CVD. At the beginning of the study, the mean doses of both products were increased slightly on switching to the fixed-dose combination. After a 6-month treatment period, significant reductions in blood pressure values were achieved and the proportion of all patients with blood pressure values above the target level of 140/90 mmHg had declined from about 30% to only 1 to 2%. It may be assumed that this success is due to the clearly documented good patient adherence to the new dosage form as a fixed-dose combination of two active substances, since no actually relevant dose increases were undertaken in the context of this study. At the same time, it should also be borne in mind that the specific information given to participants before the beginning of this study and the reference to the fact that checks were made that the drugs had actually been taken (pill counting) played a significant role in the desired outcome.
Particular significance can be attributed to the analysis results of this study by virtue of the fact that the consistent intake of the FDC of bisoprolol and amlodipine, both in hypertensive patients without comorbidities and in patients with comorbidities, was shown to yield comparable reductions in blood pressure and pulse pressure, as a result of which it is possible to reduce the significantly increased risk of cardiovascular effects in hypertensive patients with diabetes and coronary heart diseases to a relevant extent [27,28].
U. Hostalek is an employee of Merck KGaA; D. Czarnecka has given lecture for Merck KGaA and was the principal investigator of this observational study; A. Kurzeja is an employee of Merck SP. Z O.O.¸Ernst MW Koch is a consultant to Merck KGaA; The study was financed by Merck KGaA.
Download Provisional PDF Here
Aritcle Type: Research Article
Citation: Hostalek U, Czarnecka D, Kurzeja A, Koch EMW (2015) Efficacy of and Adherence to a Fixed-dose Combination of Bisoprolol and Amlodipine in Hypertensive Patients with and without Type 2 Diabetes Mellitus or Coronary Artery Disease. J Hear Health 1 (4): doi http:// dx.doi.org/10.16966/2379-769X.114
Copyright: © 2015 Hostalek U. This is an openaccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access