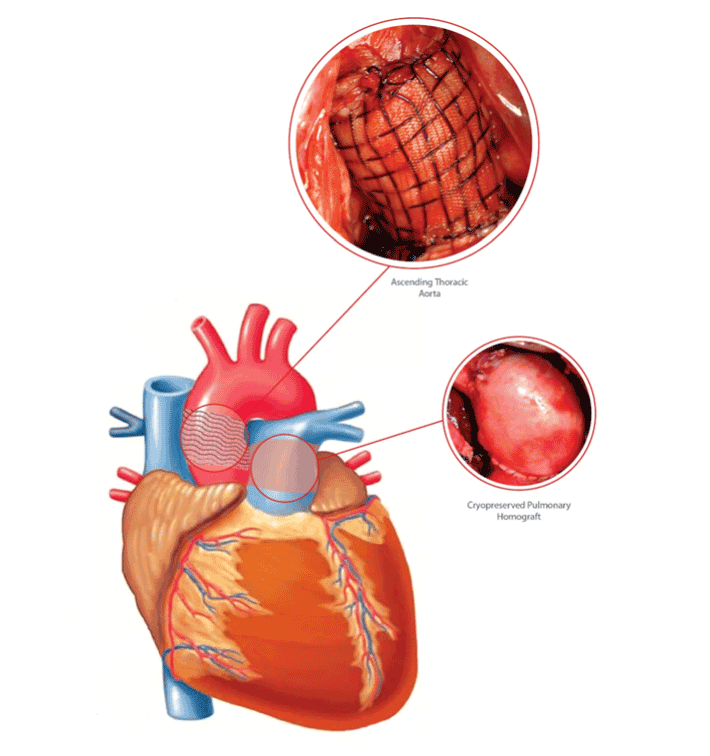
Figure 1: Use of bioresorbable scaffold in Ross procedure

Antonio Nenna1 Mario Lusini1 Massimo Chello1 Cristiano Spadaccio2 Francesco Nappi3* William Maddox1
1Department of Cardiovascular Surgery, Università Campus Bio-Medico di Roma, Rome, Italy*Corresponding author: Francesco Nappi, MD, Cardiac Surgery, Centre Cardiologique du Nord de Saint-Denis, 36 Rue des Moulins Gémeaux, 93200 Saint-Denis, France, Tel: +33 1 49 33 41 04; Fax: +33 1 49 33 41 19; E-mail: francesconappi2@gmail.com
The bioresorbable scaffolds are becoming progressively and increasingly evaluated in the practice of interventional cardiology [1,2]. They were initially developed to overcome limitations of drug eluting stents, which produce chronic local inflammation due to the permanent implantation of a foreign body, the restriction of vascular compliance, the impaired vasomotory properties due to a metal cage, and the risk of late thrombosis. Thus it became appearent the need to introduce in clinical practice a system which allows an early structural support and then dissolves over time, restoring pulsatility, cyclical strain, physiological shear stress, and mechanotransduction to the treated vessel, which ultimately resembles a native conduit [2]. The production of smart scaffolds simulating the biological assets of the natural extracellular matrix and promoting autologous stem cell growth and differentiation would allow to explore the endogenous regenerative resources avoiding the technical, financial and ethical concerns which are associated with the use of homologous stem cells [3,4]. In fact, these bioresorbable scaffolds may be used as a structural support, which might be colonized by autologous cells after the implantation and guide the formation of a totally regenerated vascular structure. The surgical correction of the diseased heart would follow the natural course of patient’s growth, with significant long-term benefits [4].
The Ross procedure is currently regarded as one of the best procedures for aortic valve disease in children and young adults, with substantial advantages in surgical outcomes, perioperative and long-term management of patients [5,6]. However, with the exception of the sub coronary technique, the major concern of this operation is the long-term dilation of the pulmonary autograft in the aortic root position [7-10]. As an attempt to overcome these limitations, a modified technique with a synthetic non-resorbable reinforcement of the pulmonary autograft has been developed, with initial promising results [11,12]. However, the late outcomes of the use of synthetic materials are gradually revealing their limitations in both clinical and structural level compared to biologic materials [13]. In fact, those materials fail to match the demand for structure growth; also, Dacron has a stiffness about 24 times greater than native aorta [14], hence the vascular compliance is poor. This adversely affects elastomechanical features and wind kessel function of the neo aortic root with retrograde effect on the aortic valve, eventually leading to its regurtitation. Furthermore, synthetic materials are known to initiate and sustain a strong inflammatory process, impairing pulmonary autograft viability and interfering with the arterialization process. This concept has been recently stressed in recently published works, which are increasingly stressing the fact that the graft viability and its biological features as the main determinants of the clinical success of the Ross operation [4].
In this context, we designed and developed a bioresorbable reinforcement tailored to give structural support to the pulmonary autograft which was stressed by systemic pressure, and to allow the structural wall adaptation for the preservation of graft viability. In our experience, we produced a bioresorbable device composed by commercially available materials, normally employed during conventional surgery, arranged in a fibrillar mesh, made of a single-layer polyglactin strengthened by an interlaced polydioxanone was easily constructed at the operative table. Polyglactin is a compound which has a great earlyresorbability properties, where as polydioxanone is a late resorbability material. The cross-linked bioprosthesis was designed to minimize radial tension with the weave of the long-term resorbable material arranged to embrace the graft allowing the multi directional growth of the pulmonary autograft. In an experimental model of Ross procedure in growing lambs, the device allowed a harmonic growth of the pulmonary autograft preventing aneurysmatic dilation of the neo-aorta [15,16] (Figure 1). Also, the biocompatible materials, avoiding the deposition of damaging inflammatory infiltrates, triggered a phenomenon of wall matrix deposition and rearrangement, which ultimately lead to the induction of an elastic remodeling of the pulmonary autograft. While controlling the enlargement of the graft under systemic conditions, the device guided the wall modification process to a mature histologically remodeled vessel. These data were confirmed by the analysis of the mechanical behavior of the system, which fitted with a mathematical model based on the Hooke law and Laplace equilibrium [16]. The integration of bioresorbable scaffold in the structure of the vessel wall limited over time the dilation process of pulmonary artery and elicited a phenomenon of histoarchitectural reorganization of the neopulmonary trunk. The remodelling process resulted in medial thickening, enhancement and differentiation of the elastic wall components, which ultimately lead to the formation of a “neovessel” with important similarities to the native aorta and pulmonary artery [15,16]. Although standard non-absorbable reinforcement prevents pulmonary autograft dilatation, the consequences of the mesh transmural migration can be deleterious, as it elicits a strong inflammatory reaction and, limiting pulmonary autograft viability, interferes with the growth and structural reorganization of the autograft.

Figure 1: Use of bioresorbable scaffold in Ross procedure
In conclusion, our pulmonary autograft reinforcement with the bioresorbable scaffold prevented vessel dilation and allowed a physiological growth of the vessel, which was guided, rather than forced, along the wall modification occurring in the pulmonary autograft postoperatively under the systemic hemodynamic conditions. The increase in elastic constituents suggests an advanced structural modification process, which may result in improved hemodynamic properties of the neo-aorta.
We have extended this concept under the circumstances involving a pressure-load conditioning of vascular structures normally under nonarterial regimens. With this in mind, we thought that the biological reinforcement might be used to prevent the complications of the arterial switch operations for simple transposition of great arteries, which mainly consist of neo-aortic root enlargement and regurgitation for the left outflow tract repair, and the onset of supravalvular pulmonary stenosis for the right ventricular to pulmonary artery reconstruction [17-21].
The use of the biological valves conduits and the pulmonary homograft conduit implanted in orthotropic and etherotopic position are still proposed for many complex congenital cardiac lesions involving the right ventricular outflow tract [22-24]. These two alternatives are not suitable in neonates with simple transposition of great arteries undergoing arterial switch operation and the use of autologous pericardium has been claimed to be the best choice to renconstruct the pulmonary trunk in these procedures. However, this technique is associated with supravalvular pulmonary stenosis, which remains a troublesome complication in the long term of simple transposition of the great arteries [25,26], as it occurs in 17% to 55% of patients after arterial switch operation with the majority of obstructions of the neopulmonary trunk confined to the neopulmonary anastomotic site [26]. Approximately 30% of the patients are suitable for a catheter-based interventions for the treatment of supravalvular pulmonary stenosis, but the disappointing results of this approach often lead to surgical reintervention [27,28]. Mechanisms underlying supravalvular pulmonary stenosis are still not fully clarified, but scar tissue formation and the existence of non-viable tissue at the anastomotic site, inadequate somatic growth of the neopulmonary trunk, and poor mobilization of both the neopulmonic root and pulmonary artery resulting in tension on the anastomosis, are presumed to be the most significant factors [17,18]. Despite the chosen surgical procedure represents an important variable in this situation, tissue viability of the neopulmonary trunk and its ability to follow the somatic growth of the vascular structures is crucial in determining supravalvular pulmonary stenosis. the reconstruction of neopulmonary trunk, aiming to recreate a vascular conduit that holds the structural architecture and the same biological potential of native pulmonary artery and prevents long-term complications, such as supravalvular pulmonary stenosis. In an animal model, reinforcement of the neopulmonary trunk with the resorbable material stimulated a remodeling process of the neopulmonary artery and it harmoniously incorporated with the neo-vessel wall. These features are important to increase vessel resistance to dilation and avoid or limit the progression of supravalvular pulmonary stenosis. Considering its biocompatibility features, the mesh did not impair the viability and physiological somatic growth of the neopulmonary artery. This idea is supported by the formation of an intact endothelial lining on the inner surface of the graft and by the consensual increase in diameter over time (Nappi et al. unpublished data). It is possible to speculate that the mechanical and biological support provided by the resorbable reinforcement allowed avoidance of both hemodynamic abnormalities at the anastomosis and scar formation, which are considered the major determinants of supravalvular pulmonary stenosis. Indeed, the mechanical support provided by the scaffold prevented excessive pressureload and shear stress at the level of the anastomosis and, at the same time, the balanced resorption of the biomaterial avoided formation of fibrotic tissue, which could have exerted detrimental effects on the elasticity and compliance of the graft, thus exasperating the vicious circle known to lead to neointimal hyperplasia and supravalvular pulmonary stenosis.
We, therefore, have introduced the use of a similar approach in the reconstruction of neopulmonary trunk, aiming to recreate a vascular conduit that holds the structural architecture and the same biological potential of native pulmonary artery and prevents long-term complications, such as supravalvular pulmonary stenosis. In an animal model, reinforcement of the neopulmonary trunk with the resorbable material stimulated a remodeling process of the neopulmonary artery and it harmoniously incorporated with the neo-vessel wall. These features are important to increase vessel resistance to dilation and avoid or limit the progression of supravalvular pulmonary stenosis. Considering its biocompatibility features, the mesh did not impair the viability and physiological somatic growth of the neopulmonary artery. This idea is supported by the formation of an intact endothelial lining on the inner surface of the graft and by the consensual increase in diameter over time (Nappi et al. unpublished data). It is possible to speculate that the mechanical and biological support provided by the resorbable reinforcement allowed avoidance of both hemodynamic abnormalities at the anastomosis and scar formation, which are considered the major determinants of supravalvular pulmonary stenosis. Indeed, the mechanical support provided by the scaffold prevented excessive pressureload and shear stress at the level of the anastomosis and, at the same time, the balanced resorption of the biomaterial avoided formation of fibrotic tissue, which could have exerted detrimental effects on the elasticity and compliance of the graft, thus exasperating the vicious circle known to lead to neointimal hyperplasia and supravalvular pulmonary stenosis.
We have designed and created a device for the rapid application of bioresorbable scaffold in Ross procedure and in neopulmonary artery surgery. This device consists of an internal resorbable scaffold made with Polydioxanone (PDS) and an external non-resorbable layer of expanded Polytetrafluoroethylene (ePTFE) specifically designed to absorb mechanical stress and assist graft’s growth. The device is presented in Figure 2 and Figure 3. The employed materials are commercially available and they have been assembled on the basis of their biophysical and elastomechanical properties. Polydioxanone layer was organized in a frame of hexagonal cells strengthened by external armour of expanded polytetrafluoroethylene, which was joined externally to the bioreabsorbable material and encompasses a multitude of longitudinal lateral strips, having an auxetic behaviour, and a number of transverse wires, which connect each longitudinal strip with the two neighboring. The layer of auxetic material was realized as a deformable matrix and applied to the bioresorbable in order to orient in the same direction the polymer chains and thus make the overall structure more compact. The layer of bio-absorbable material was designed to minimize radial tension and was constructed to embrace the root of the great vessels allowing its multidirectional growth.
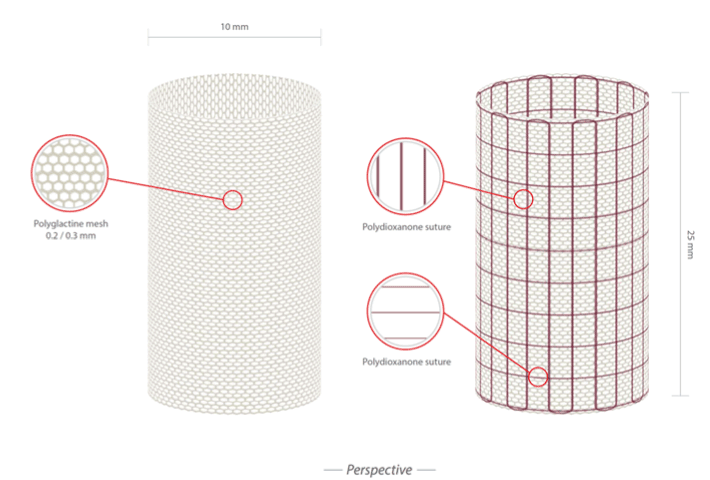
Figure 2: Perspective view of the bioresorbable scaffold
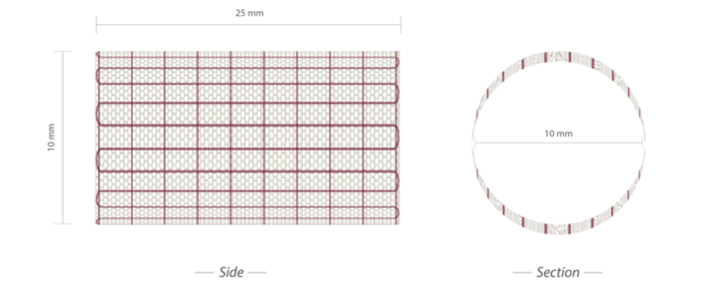
Figure 3: Side and section view of the bioresorbable scaffold
In the near future, off-the-shelf available smart bioprostheses promoting self-endothelialization with patient’s cells could represent a solution that would allow for autologous vascular tissue regeneration rather than its mere replacement by an artificial prosthesis. The idea of the use of bioresorbable scaffolds in adult and paediatric cardiovascular surgery is extremely challenging and we hope than would became part of routine procedures for most cardiovascular surgery Centers.
Acknowledgements: none.
Conflicts of Interest: none.
Download Provisional PDF Here
Aritcle Type: Short Communication
Citation: Nenna A, Lusini M, Chello M, Spadaccio C, Nappi F (2015) The use of Bioresorbable Scaffolds in Cardiovascular Surgery. J Heart Health 1(2): doi http://dx.doi. org/10.16966/2379-769X.108
Copyright: © 2015 Nenna A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access