Abstract
Atrial Fibrillation (AF) increases the risk of embolic Cerebrovascular Accidents (CVA) with the thrombus predominately originating in the Left
Atrial Appendage (LAA). In patients unable to take oral anticoagulation, closure or exclusion of the LAA can be performed to mitigate the risk of
stroke. We present a case of a patient who underwent LAA closure with an epicardial suture delivery device, and developed a late post procedure
pericardial effusion despite perioperative anti-inflammatory therapy. This was successfully treated conservatively with oral steroids, negating the
need for further invasive therapy with pericardiocentesis.
Keywords
Atrial fibrillation; Anticoagulation; Left atrial appendage Closure; Pericardial effusion
Introduction
Atrial Fibrillation (AF) has an estimated prevalence of 1.5-2% currently
[1], and it is expected to increase from a prevalence of 5.2 million cases in
2010 to an estimated 12.1 million cases in 2030 [2]. AF has been associated
with an increased risk of embolic Cerebrovascular Accidents (CVA).
In patients with non-valvular AF, the risk of having an embolic CVA is
increased 5.6 fold. This incidence increases with age and with increasing
comorbidities [3,4]. Warfarin, a vitamin K antagonist and, more recently,
Novel Oral Anticoagulants (NOACs) are prescribed to mitigate the risk
of CVA in patients with AF [5]. Warfarin has been shown to reduce the
risk of embolic CVA in AF patients by up to 62% [6]. Stroke prophylaxis
with NOACs has shown to be non-inferior and, in some cases, superior to
warfarin therapy in this patient population. Major bleeding complications
are lower with the NOACs as compared to warfarin for the prevention of
stroke in AF patients [7-9].
Unfortunately due to increased bleeding risks, some patients cannot
tolerate anticoagulation therapy. In patients with non-valvular AF, 91%
of left atrial thromboses were isolated to the Left Atrial Appendage (LAA)
[10]. Due to this, there has been a great interest in LAA closure devices
in AF patients who are poor candidates for oral anticoagulation but are at
an increased risk for CVA. LAA closure using the LARIAT suture delivery
device (Sentre HEART Inc., Redwood City, California) can be performed
on patients with a high risk for CVA with or without contraindications to
anticoagulation. Greater interest has been placed on high risk patients for
CVA who are not candidates for anticoagulation [4].
We present a case report on a patient who underwent LAA closure
using the LARIAT suture delivery device, as she was deemed too high
risk for oral anticoagulation. Post procedurally, the patient developed a
late pericardial effusion seen by Trans-Thoracic Echocardiogram (TTE).
Due to patient’s reluctance to undergo pericardiocentesis, she was treated
conservatively with steroids with resolution of her pericardial effusion.
Case
An 81 year old female with a past medical history of frequent
falls complicated by Subdural Hematoma (SDH) and concussion,
hypothyroidism, and dementia presented with paroxysmal AF with a
CHADSVASC of 3 as a referral for evaluation for LAA closure. She was
previously treated with warfarin, but in recent years, she was noted to
have frequent falls with one resulting in a SDH subsequently leading to
her warfarin being discontinued. LAA closure using the epicardial suture
delivery device was discussed with the patient and her daughter including
the risks, benefits, and alternatives. Both the patient and her daughter
expressed interest in proceeding with pre-procedure workup and to
undergo the procedure.
Pre-procedure cardiac Computerized Tomography (CT) was obtained,
which showed the patient’s LAA size and orientation were amenable to
closure using the epicardial suture delivery device. Pre-procedure TransEsophageal
Echocardiogram (TEE) was obtained, which did not show any
evidence of LAA thrombus. She subsequently underwent closure of the
LAA using a percutaneous subxiphoid approach to deliver a permanent
suture around the epicardial base of the LAA as previously well describe
[4]. The procedure was successful and the patient tolerated it without
difficulty. A post-procedure TTE was obtained, which showed a trivial
anterior pericardial effusion (Figure 1A). Output from the pericardial
drain was minimal over the next 24 hours and was removed on postprocedure
day 1. She was subsequently discharged home with twice daily
colchicine and outpatient follow up was arranged.
The patient returned to the Emergency Department (ED) three hours
after being discharged from the hospital with shortness of breath with
ambulation, light headedness, and sudden sharp substernal chest pain
associated with vomiting. In the ED, she was noted to be hypoxic requiring
a non-re breather face mask. CT angiography of chest was obtained, which
showed extensive bilateral Pulmonary Emboli (PE). Patient was not
deemed a candidate for systemic Tissue Plasminogen Activator (tPA) and
was placed on systemic intravenous heparin. She underwent emergent
successful thrombectomy of the left main pulmonary artery with residual
thrombus burden in the sub segmental right pulmonary arteries. An
Inferior Vena Cava (IVC) filter was also placed at the end of the case.
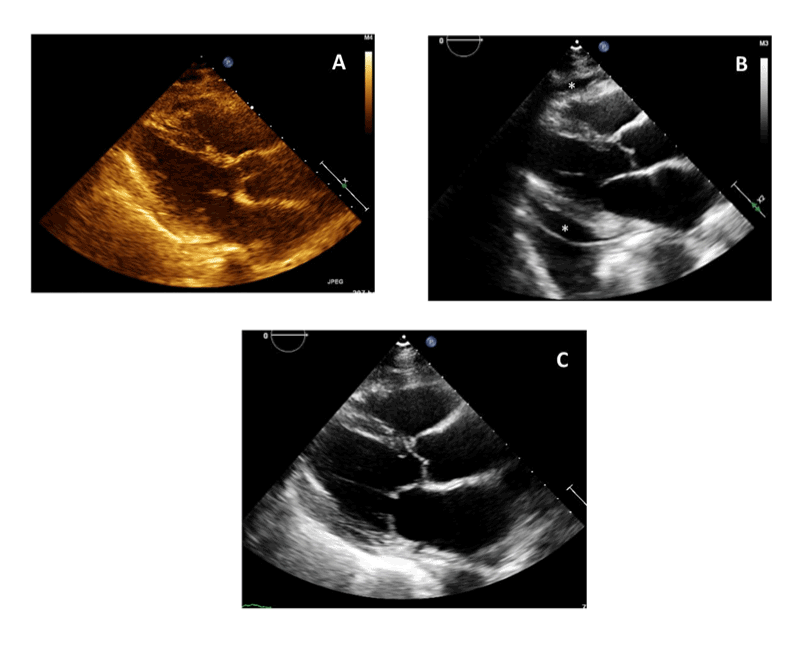
Figure 1: Transthoracic Echocardiogram (TTE). Parasternal Long axis
view.
(A): Post-procedure Day 1 TTE.
(B): 3 weeks Post-Procedure TTE showing moderate pericardial effusion (effusion shown with asterisks).
(C): TTE after 1 week of steroid initiation showing resolution of pericardial effusion.
Post procedure TTE was obtained, which showed an increase in
Right Ventricular (RV) size and reduced function but did not show any
pericardial effusion. Patient was subsequently transitioned to apixaban
for acute treatment of a pulmonary embolus and was discharged home
with Electrophysiology (EP) outpatient follow up. The patient presented
to the EP clinic for her post procedure visit complaining of a cough that
was exacerbated by lying in the supine position. She was no longer taking
colchicine at the time of her clinic visit, although this had been prescribed
for a period of six weeks after the procedure. A TTE was obtained,
which revealed a moderate circumferential pericardial effusion (Figure
1B). A pericardiocentesis was offered to the patient, but patient refused
to undergo any further procedures. She was restarted on colchicine and
started on oral lasix with follow up in 1 week.
During her one week follow up, the patient continued to have
worsening cough and a repeat TTE showed stable moderate pericardial
effusion without tamponade physiology. Pericardiocentesis was again
discussed with the patient, but she again declined. A tapering dose of
oral methyl prednisolone was then initiated. She was followed up in one
week with a repeat TTE, which showed almost complete resolution of her
pericardial effusion (Figure 1C). Her symptoms of positional dyspnea,
fatigue, and decreased exercise tolerance had resolved as well. Repeat
echocardiography three months later demonstrated that the effusion did
not recur.
Discussion
As mentioned above, the incidence of atrial fibrillation is projected
to increase over the next decade. With an aging population, a significant
number of those patients will have contraindications to anticoagulation
for CVA prophylaxis. As a consequence, non-pharmacologic treatment for
the prevention of CVA in this population will continue to become more
common. Currently, there is great interest in percutaneous, epicardial
LAA closure using the LARIAT suture delivery device in patients who are
not candidates for anticoagulation.
In patients who undergo this procedure, post-procedure pericardial
effusion is relatively common with the incidence being between 10% to
44% [11,12]. In one study, 25% of the pericardial effusions were attributed
to LAA perforation and laceration, 25% were attributed to pericardial
access (right ventricular perforation or other bleeding during pericardial
access), and 50% did not have an attributable cause [11]. The definite
therapy for LAA or RV perforation would be drainage of the pericardial
space, which is usually not an issue as a pericardial drain is left in place
post procedure. Little is known about the 50% of cases with no attributable
cause to the effusion.
In our patient, it is unlikely that she had LAA perforation or laceration
or RV perforation as her pericardial drain output was minimal. When
she represented with a PE, a repeat TTE was obtained approximately
72 hours after her initial procedure and did not show any evidence of a
pericardial effusion. The late presentation of the effusion effectively rules
out mechanical injury as the etiology of the effusion.
The pericardial effusion that our patient developed post procedure
was likely inflammation mediated and similar to that seen in surgical
patients with Post Pericardiotomy Syndrome (PPS). Approximately 88%
of PPS patients develop pericardial effusions. The usual diagnosis of the
pericardial effusion is 3-4 weeks post- procedure [13], which is similar to
our patient, who was noted to have pericardial effusion three weeks postprocedure.
Our patient was placed on colchicine without improvement in
her effusion. Steroids were then initiated, which resolved her effusion as
well as her symptoms. In patients with PPS and pericardial effusions, those
who are on colchicine and an anti-inflammatory medication (NSAIDs or
steroids) have a lower risk of adverse outcomes and a less incidence of
further procedures (e.g. pericardiocentesis) [13].
Colchicine has been shown to reduce the incidence of pericardial
effusion in PPS patients [14]. Our patient was discharged on colchicine
post procedure; however, after being hospitalized for a PE and on her
subsequent EP clinic follow up, she was no longer taking colchicine. This
may explain why our patient did not have a pericardial effusion on the
TTE obtained when she was diagnosed with a PE, but it later developed
and was seen on a repeat TTE three weeks after the procedure. In
addition, once our patient was started on anticoagulation, it is possible
that she had small amount of bleeding in the pericardial space owing to
the recent instrumentation of this area. Blood has been shown to be a
potent inducer of inflammation in the pericardial space and this may well
have contributed to her pericardial effusion as well.
We propose that the 50% of non-attributable pericardial effusions
seen patients undergoing LAA closures using an epicardial, percutaneous
suture delivery device may be secondary to pericardial inflammation
with similar pathophysiology to PPS. Other hypothesis would be that the
ligation of the LAA itself could be the source of pericardial inflammation
as this tissue dies and is resorbed over time. At times, physicians have
been reticent to use steroids in this patient population owing the concern
for an increased risk of bleeding. Our patient was treated similarly to
PPS patients with steroids with resolution of her pericardial effusion. In
patients who undergo LAA closure with LARIAT device, steroids may
have a role in treating pericardial effusions, especially when conservative
management is preferred. Further studies need to be done to assess the
role of colchicine in preventing post LARIAT procedure pericardial
effusions and to assess the role of steroids in non-traumatic (i.e. LAA or
RV perforations) post procedure pericardial effusions.
Author contributions
Dr. Shah and Dr. Maddox have contributed equally to the manuscript.
Both authors are thoroughly familiar with the case and the data, have
thoroughly read the manuscript, are responsible for the contents, and
have approved the manuscript for submission.
Article Information
Article Type: Case Report
Citation: Shah RR, Maddox W (2015) Steroid
Responsive Pericardial Effusion after
Percutaneous Epicardial Closure of the Left
Atrial Appendage. J Hear Health 1 (2): doi
http://dx.doi.org/10.16966/2379-769X.106
Copyright: © 2015 Shah RR. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction
in any medium, provided the original author and source are credited.
Publication history:
Received date: 09 June, 2015
Accepted date: 19
June, 2015
Published date: 22 June, 2015


