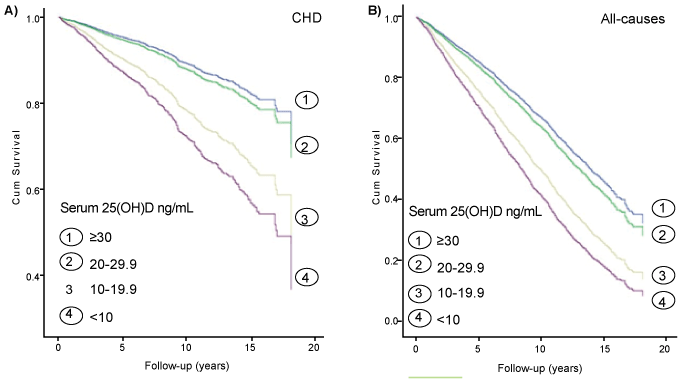
Figure 1:Survival function of patients with T2DM died from CHD (Figure A) and all-causes (Figure B)

Longjian Liu*
Department of Epidemiology and Biostatistics, and Department of Environmental and Occupational Health, Drexel University School of Public Health, Philadelphia, PA, 19104, USA*Corresponding author: Longjian Liu, MD, PhD, MSc (LSHTM), FAHA, Interim Chair of the Department of Environmental & Occupational Health, Associate Professor, Department of Epidemiology & Biostatistics, Drexel University School of Public Health, RM 515, Nesbitt Hall, 3215 Market ST, Philadelphia, PA 19104, USA, Tel: 267-359-6049, 267-886-5138; E-mail: Longjian.Liu@Drexel.edu
The study aimed to examine the joint predicting effect of serum 25(OH) D (a biomarker of vitamin D level in blood) and C-reactive Protein (CRP) concentration on the risk of mortality from Coronary Heart Disease (CHD) and all-causes in patients with type 2 Diabetes Mellitus (T2DM) using data from the third National Health and Nutrition Examination Survey (NHANES III). Of 14965 subjects aged ≥ 30 years who participated in the NHANESIII, 2146 patients with T2DM at baseline (1988-1994) were followed up through December 31 of 2006. The associations of baseline serum 25(OH) D and CRP with outcomes were examined prospectively using Cox’s hazard proportional regression models. The results show that during a median follow-up of 12.3 years, 1257 (58.6%) of the patients with T2DM died, and 370 deaths were from CHD. Multivariate adjusted Cox’s models indicate that decreased 25(OH) D level significantly predicted risk of death. The corresponding hazard ratios for the risk of CHD mortality among those with serum 25(OH) D levels 20-29.9, 10-19.9, and <10 ng/mL were 1.35, 1.38 and 2.19, as compared to those with 25(OH) D ≥ 30 ng/mL, respectively. Similar associations between decreased 25(OH) D and risk of all-cause mortality were observed. Furthermore, a joint effect of decreased 25(OH) D and increased CRP significantly predicted an increased risk of CHD and all-cause mortality. In conclusion, using data from a nationally representative and longitudinal survey, findings from the study suggest that decreased serum vitamin D and increased CRP concentrations significantly predicted the risk of CHD and all-cause mortality in patients with T2DM.
Prospective Study; Vitamin D; Inflammation; Mortality
Levels of serum 25-hydroxyvitamin D [25(OH) D, a biomarker of serum vitamin D concentration] vary considerably among individuals mainly because of differences in sun exposure and skin color and the presence of risk factors such as, cigarette smoking, obesity, Diabetes Mellitus (DM) or other co morbidities. Hypovitaminosis Dishighly prevalent worldwide. Several population-based studies have reported a cross-sectional association between decreased serum 25-hydroxyvitamin D (25(OH) D) concentration and increased prevalence of Coronary Heart Disease (CHD) in the general population and in patients with type 2 DM (T2DM) [1-5]. However, evidence from studies with a prospective longitudinal study design is limited [6-8]. Although previous studies suggested that decreased serum 25(OH) D and increased serum C-reactive protein (CRP, a biomarker of systematic inflammation) were independently associated with risk of T2DM, none of these studies examined whether there is a significant joint effect of serum 25(OH) D and CRP concentration on the risk of CHD mortality in patients with T2DM [9,10]. The present study aimed to test two hypotheses. First, there is a significant longitudinal association of decreased serum 25(OH) D concentration with an increased risk of CHD and all-cause mortality in patients with T2DM. Secondly, there is a significantly joint or interactive effect of decreased 25(OH) D and increased serum CRP levels on the risk of CHD and all-cause mortality. To test these hypotheses, data from the third National Health and Nutrition Examination Survey (NHANES III), a nationally representative longitudinal study, was used in the study [11].
The baseline NHANES III was conducted by the National Center for Health Statistics (NCHS) of the Centers of Disease Control and Prevention (CDC) between October 1988 and October 1994, gathering information representing the health and nutritional status of the non-institutionalized civilian US population aged two months and older. The study consisted of interviews, physical examinations, and data from blood sample analysis. Household interviews were conducted prior to the examination to collect demographic, medical history, and health behavior information. Physical examinations were carried out in Mobile Examination Centers (MEC). Blood samples were obtained at MEC for measurements of serum biomarkers. Fasting glucose and lipid profiles were measured using routine standardized approaches, serum 25(OH) D concentration was measured using a radio immunoassay kit (DiaSorin). Serum CRP concentration was measured using a latex-enhanced nephelometry method. The quality of control for all survey procedures and lab tests were conducted on the basis of the NCHS criteria. The NHANES III Mortality-Linked Study was done by the NCHS working with the State’s Offices of Vital Statistics to link NHANES III data with death certificate records using the National Death Index (NDI) [11]. The currently released Mortality Linked File records NHANES III baseline participants’ survival status or causes of death from baseline until the end of December 2006. The NHANES III has been approved by the CDC Institutional Review Board (IRB). Data obtained from the NCHS for the present study are de-identified for the purpose of participants’ private information security [11,12]. The present analysis included participants who were aged 30years and older at baseline, as subjects in these age groups have higher prevalence of T2DM and CHD risk than those aged less than 30.T2DM was defined using the American Diabetes Association criteria for those who had fasting glucose ≥126mg/ dl or HbA1c ≥ 6.5% or those who were using anti-diabetic medication or that had been told by a physician that she/he had diabetes. Of 14,965 participants aged ≥ 30 years, 2,146 were classified as having T2DM and are included in the present report.
In the study, although there is no standard threshold of the classification of serum 25(OH) D level, for the purpose of clinical practice and reference to the most commonly used cutoff points, serum 25(OH) D Concentrations were divided into four groups: (1) serum 25(OH) D <10 ng/ml (deficiency), (2) serum 25(OH) D 10-19 ng/ml (insufficiency II), (3) serum 25(OH) D 20-29 ng/ml (insufficiency I), and (4) serum 25(OH) ≥ 30 ng/ml (normal) [13-16]. Mortality from CHD was defined according to International Classification of Disease Revision 10 (ICD-10: I11, I20-I25). All-cause mortality included those who died for any reason.
A serial analysis was conducted. First, the basic characteristics of the study participants were described according to the participants’ survival status. Differences between alive and deceased were tested using Chisquare test for categorical variables, and t-test for continuous variables. Second, hazard ratios (HRs) and HRs 95% confidence interval (CI) of serum 25(OH) D for risk of mortality from CHD and all-causes were estimated using Cox’s proportional hazard regression (Cox’s) models. To control potential confounders, three sets of multivariate Cox’s models were conducted: (1) Model 1: adjustment for age (year), sex (male vs. female), and race/ethnicity (White, Black, Hispanic and others); (2) Model 2: adjustment for covariates used in Model 1 plus education level(recoded 0,1 and 2 for those with more than high school, high school, and less than high school level, respectively), smoking status (current smokers vs. non smokers), and body mass index (BMI, kg/m2); (3) Model 3: adjustment for covariates used in Model 2 plus serum CRP concentration. Interaction of serum 25(OH) D and CRP concentration on risk of CHD and all-cause mortality was tested. Finally, survival function of diabetic patients across 4 different serum 25(OH) D levels (<10, 10-19.9, 20-29.9 and ≥ 30 ng/mL) for risks of CHD and all-cause mortality were examined using Kaplan Meier survival curve analysis. Joint effects of serum 25(OH) D (classified as three groups: <10, 10-19.9, and ≥ 20 ng/mL) and CRP (≤ 3 vs. >3 mg/dL) concentrations on changes in mortality rates from CHD and allcauses were examined and depicted.
All statistical analyses were performed using SAS version 9.1(SAS Institute, Cary, NC). Analysis for complex sample surveys were used in order to take in to consideration the NHANES III’s multiple sampling survey designs [17]. In Cox’s regression analysis, the proportional hazard assumption was examined using the plots of the log (-log) survival curves and Schoenfeld residuals methods [17,18]. A two-sided P value ≤0.05 was considered as having statistical significance in all data analysis.
During a mean of 10.38 years of follow-up (median: 12.3 years), 1,257 of 2,146 patients with T2DM died (58%). Of them, 370 died from CHD. Subjects who were older, had lower BMI and serum 25(OH)D, and higher serum CRP concentration had significantly higher mortality from all-causes than their corresponding counterparts (Table 1). The diseasespecific mortality from CHD in patients with DM who had serum 25(OH) D level <10, 10-19.9, 20-29.9, and ≥ 30 ng/mL were17.23%, 14.39%, 13.57%, and 12.58%, respectively (test for trend of the association between increased 25(OH)D levels and decreased CHD mortality rates:, p<0.01). The mortality from CHD in patients with DM who had serum CRP levels >3, 1-3, and <1 mg/dL were 26.21%, 12.27%, and 13.49%, respectively (p<0.01).
Cox’s model analyses (Table 2) indicate that patients with serum 25(OH) D <10 ng/mL had 2.16 and 2.32 times higher risk of mortality from CHD and all-cause than those with serum 25(OH)D ≥ 30 ng/ml (model 2), these HRs slightly increased after adjustment for CRP (model 3). Figure 1 shows that decreased serum 25(OH) D levels were significantly associated with decreased probabilities of survival in patients who died from CHD (Figure 1A) and all-cause mortality (Figure 1B). There were non-significant interaction effects of serum 25 (OH) D and CRP on CHD mortality (p=0.112), and on all-cause mortality (p=0.89).
Figure 2 depicts that there was a significant joint effect of serum 25(OH) D and CRP concentrations on the risks of CHD (Figure 2A) and all-cause mortality (Figure 2B). Diabetic patients who had the lowest serum 25(OH)D (<10 ng/mL) and the highest CRP levels (>3 mg/dL),as compared to those who had the highest 25(OH)D (≥ 20 ng/mL), and the lowest CRP (≤3 mg/dL), had the highest mortality from CHD (36.9% vs. 13.2%, p<0.001), and from all-cause (93.3% vs. 46.0%, p<0.001) during an average of 12.3 years of follow-up.

Figure 1:Survival function of patients with T2DM died from CHD (Figure A) and all-causes (Figure B)
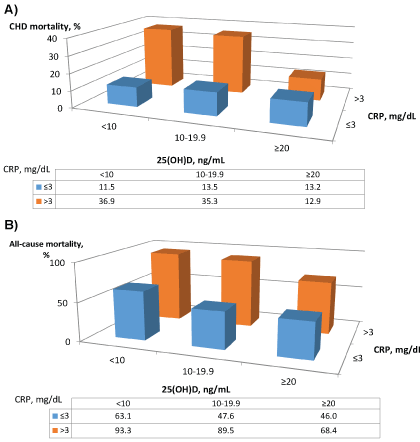
Figure 2: Joint effects of serum 25(OH) Dand CRP concentrations on CHD (Figure A) and all-cause (Figure B) mortality
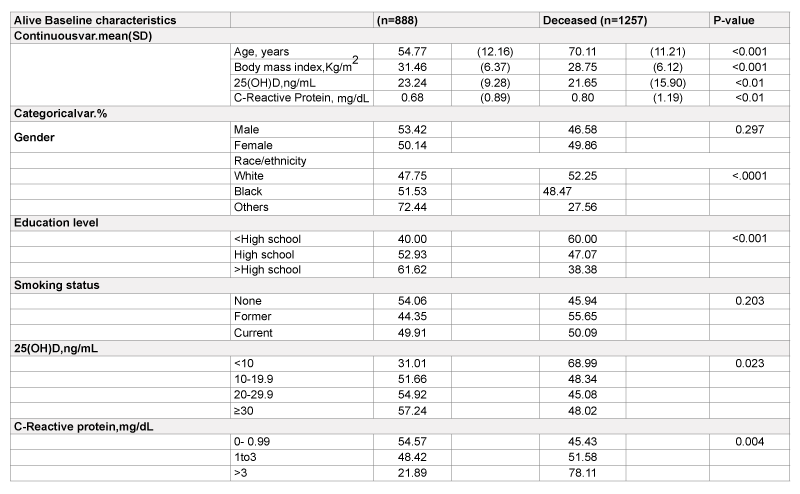
SD: Standard deviation.BMI:Body mass index.
25(OH)D: Serum25-hydroxyvitaminD.a
Table 1: Characteristics of participants with diabetes mellitus at baseline 1988-1994 and mortality by the end of 2006
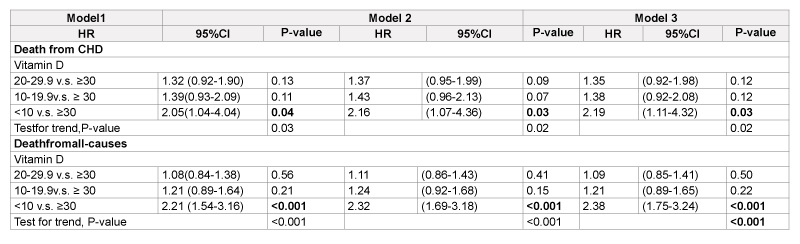
CHD: Coronary heart disease.
Model1: Adjusted forage, gender,race/ethnicity.
Model2: Adjusted forModel1 pluse ducation, smoking status and BMI,kg/m2.
Model3: Adjusted forModel2 plus serum C-reactive protein (0-0.99,1-3,and>3 mg/dl).
Table 2: Hazardratios (95%CI) of vitamin deficiency for risk of mortality from coronary heart disease and all-causes in patients with diabetes,1988-1994 baseline, and 2006 mortality follow-up
The main findings of the present study are that low serum 25(OH) D concentration was significantly associated with an increased risk of mortality from CHD and all-causes in patients with T2DM, and that there were significant joint effects of low serum 25(OH) D and increased CRP concentrations on the risks of CHD and all-cause mortality. These results are consistent with most previous reports, but add new evidence to the literature by highlighting a dose-response and longitudinal association between the predictors and outcomes using data from a nationally representative survey.
The prevalence of T2DM is growing rapidly worldwide, with total prevalence is projected to increase to 21% of the US adult population by 2050. Diabetes and complications causes substantial loss in length and quality of life. In 2007, diabetes cost the US in excess of $174 billion [19,20]. The relationships between vitamin D level and the disease progression and vascular complications have been reported by several studies [1,7]. For example, Melamed et al. using data from baseline NHANES III, found across- sectional association between low vitamin D level and all-cause mortality [1]. Joergensen et al. using data from a longitudinal follow-up study in patients with T2DN (n=289), found severe vitamin D deficiency (defined as the lower 10th percentile, serum 25(OH) D <5.57 ng/mL) to be associated with increased risk of cardiovascular disease and all-cause mortality [7]. However, evidence from large-scale and longitudinal studies is limited. Results from the present study suggest that serum 25 (OH) D deficiency and insufficiency may have a long-term impact on the risk of CHD and all-cause mortality. In addition to an independent effect of serum 25(OH) D levels on the study outcomes, a significant joint effect of decreased serum 25(OH) D and increased CRP levels on the mortality was demonstrated, which shows a dose-response and synergy effects on the study outcomes. Although further studies, such as clinical trials, are needed to confirm these findings, these results add to our understanding of the potential independent and joint effects of serum vitamin D and inflammation on the risk of CHD and all-cause mortality in patients with T2DM. It may offer new insights in to the prevention of diabetic complications using a very cost-effective approach through improving serum vitamin D concentration and control CRP, if this cause-effect association is confirmed.
The mechanisms by which decreased serum vitamin D level increase the risk of CHD and mortality in patients with T2D Mare still largely unknown. Some studies have suggested that vitamin D deficiency and insufficiency may lead to insulin resistance and beta cell dysfunction, [21] and may increase risk of inflammation, and subsequently lead to an increased risk for CHD and mortality [1,21-23]. Results from few clinical trials that had small sample sizes observed an increased serum vitamin D levels to be associated with a reduction of the risk of cardiovascular complications in patients with T2DM [15,24]. Findings from these previous studies and from the present study call for further large-scale randomized clinical trials to confirm results from observational studies.
The present study has several advantages. First, the analysis is on the basis of data from a large-scale nationally representative observational survey. Therefore, they offer the potential of generalizability to community-based population studies. Secondly, findings of the study are derived from a sophisticated analytical design using a prospective analysis approach. The associations of decreased serum 25(OH) D with increased risks of CHD and all-cause mortality support the current cause-effective hypothesis.
However, several limitations should be kept in mind when interpreting the present results. First, the baseline serum 25(OH) D and CRP concentrations were measured only once. It is unknown if there was any change of their concentrations during the follow-up period. This timevariation in predictors’ concentration, such as seasonal change, may lead to either an under or over estimation of the study associations of interest. Secondly, the study is unable to test the risk of decreased serum 25(OH) D and/or increased CRP concentrations for the incidence of CHD because the NHANES III has had follow-up data for mortality only.
Despite therefore mentioned limitations, the present study, using a nationally representative sample, adds new evidence to the body of literature on the prediction of serum biomarkers of 25(OH) D and CRP for the risk of mortality in patients with DM.
Data used in this study is from the Centers for Disease Control and Prevention (CDC) and National Center for Health Statistics (NCHS). The views expressed in this paper are those of the author and do not necessarily represent the views of the CDC and NCHS.
None.
Article Type: Research Article
Citation: Longjian L (2015) Joint Effects of Serum 25(OH) D and C-Reactive Protein Concentration on Coronary Heart Disease and All-cause Mortality in Patients with Diabetes Mellitus. J Heart Health 1 (1): http://dx.doi.org/10.16966/2379-769X.105
Copyright: © 2015 Longjian L. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access