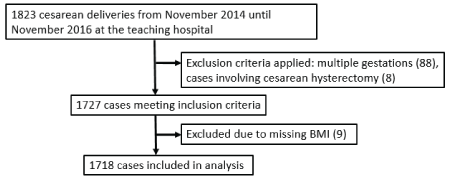
Figure 1: Flow diagram of participant selection process.


Jared T Roeckner* Luis Sanchez-Ramos Andrew M Kaunitz
Jacksonville Department of Obstetrics and Gynecology, Division of Maternal Fetal Medicine, University of Florida College of Medicine, Jacksonville, Florida, United States*Corresponding author: Jared T Roeckner, Jacksonville Department of Obstetrics and Gynecology, Division of Maternal Fetal Medicine, University of Florida College of Medicine, Jacksonville, Florida, United States, Tel: 904-244-3408; E-mail: jared.roeckner@jax.ufl.edu
Objective: To test the hypothesis that increased maternal body mass index (BMI) is associated with longer operative and anesthesia times, increasing blood loss, and lower APGAR scores.
Methods: Retrospective cohort study of 1718 pregnant women undergoing cesarean delivery at a teaching institution from 2014-2016 grouping patients into four BMI categories: <30kg/cm2 , 30-39.9, 40-49.9, ≥50. Primary outcomes included operative times (total, incision-todelivery, uterine-to-delivery, closing) and spinal anesthesia time. Secondary outcomes included blood loss, APGAR score, length of stay, and type of uterine incision. Outcomes were compared using ANOVA or Kruskal-Wallis and Wilcoxon tests.
Results: Increasing BMI was associated with significant greater operative and spinal anesthesia time. Median total operative time and interquartile range in minutes increased as follows: 90 (80-109), 100 (84-116), 110 (94.5-128), 126 (112-146) for the four BMI categories, respectively (p<0.001). Incision-to-delivery time increased by 2, 4, 4 minutes for BMI 30-39.9, 40-49.9, and ≥50, respectively (p<0.001). Average blood loss was significantly increased by 51 ml (BMI 30-39.9), 129 ml (BMI 40-49.9), and 219 ml (BMI ≥50). Classical uterine incision significantly increased with higher BMI. APGAR scores and maternal transfusion were similar.
Conclusion: Increasing BMI is associated with longer operative and anesthesia times, increased blood loss, and increased classical uterine incisions.
Obesity; Cesarean delivery; Operative time; Blood loss
Obesity affects one third of women and creates challenges for the management of labor. Obesity is classified by body mass index (BMI) as follows: Class I (30-34.9), Class II (35-39.9), Class III (>40) and is a risk factor for fetal macrosomia, gestational diabetes mellitus, hypertension, preeclampsia, gestational hypertension, and cesarean delivery [1-5]. Additionally, obesity complicates the estimation of fetal weight and fetal presentation, the placement of neuraxial anesthesia, the success of labor induction, and the completion of cesarean delivery [6-8]. Postpartum wound breakdown, wound infection, venous thromboembolism, and maternal mortality are more common in obese patients [9-12].
Several studies have shown that operative times increase as BMI increases [9-15]. Girsen et al. [14] showed that the incision-to-delivery interval was increased by three minutes in a study that included 21, 372 women (46.5% obese and 14% morbidly obese). In a smaller study by Conner et al. [13], increasing obesity was associated with increasing time from skin incision to infant delivery. This study found a dose response relationship with the longest operating times found in the group with BMI >50. These studies did not assess closing time and time for neuraxial anesthesia.
We sought to investigate the relationship between maternal BMI and (1) operative times (total, incision-to-delivery, closing); (2) time for spinal anesthesia administration; (3) blood loss during the procedure; (4) incidence of low APGAR scores (<7 at 5 minutes); and (5) type of uterine incision.
We performed a retrospective cohort study of all consecutive cesarean deliveries at a tertiary urban teaching facility during a 24 month period from 2014 to 2016. The facility performs about 3000 deliveries annually. Obstetric and anesthesia services are provided by a combination of residents and attending physicians. Institutional Review Board approval was obtained prior to the start of the study. Inclusion criteria included women with a singleton gestation delivered via cesarean during the study interval without regard to gestational age, indication, history of prior cesarean delivery, or labor prior to cesarean delivery. Exclusion criteria included women with multiple gestations (88) or cases involving cesarean hysterectomy (8), or missing BMI (9). It was calculated that using a power (0.8) and type 1 error rate of 0.05, the charts of approximately 150 patients would need to be reviewed to show a 10 minute increase in operative time.
Demographic information was extracted from the electronic medical record using a combination of chart review and electronic query of discrete data points within the electronic medical record. Data obtained included maternal age, gestational age, history of prior cesarean, indication for cesarean (grouped as non-reassuring fetal tracing, labor dystocia, or other), primary or repeat cesarean, length of stay, completion of tubal ligation at time of surgery, emergency nature of the procedure, and anesthesia type. BMI was calculated at time of admission and grouped into four categories. BMI <30 served as the reference group and obese women were stratified into three comparison groups: BMI 30-39.9, 40- 49.9, ≥50.
The primary outcomes were operative times including (1) total operating time (time from room entry to room exit), (2) skin incisionto-delivery interval, (3) uterine incision-to-delivery interval, (4) closing time (interval from delivery of the infant to closure of the skin), (5) spinal anesthesia time (interval from anesthesia timeout to skin incision). These times are routinely recorded in the chart by the operating room nurse.
Secondary outcomes included blood loss as indicated on the operative report (estimated blood loss or quantitative blood loss), blood loss >1500 ml, maternal blood transfusion, type of uterine incision (defined as low transverse, classical, other), length of stay, and APGAR score <7 at 5 minutes.
Baseline characteristics were compared among the study groups using one-way ANOVA for continuous variables and chi-squared test for categorical variables. Continuous variables were tested for normal distribution using histograms and the Shapiro-Wilk goodness of fit test. Normally distributed variables were compared among groups using oneway ANOVA test with Tukey post-hoc analysis. Nonparametric testing using Kruskal-Wallis and Wilcoxon comparisons were used for data not normally distributed. Bivariate analysis was used to identify potentially confounding variables. A multiple linear regression model was used to test the independent relationship of total time, incision-to-delivery interval, and time for spinal anesthesia compared to BMI while controlling for confounders including emergency cesarean, repeat cesarean, and general anesthesia. Backward step-wise analysis was used to reduce the number of variables in the model. All statistical analyses were completed using JMP Pro 13.0 (Cary, NC). Differences with p<0.05 were considered significant.
During the study period, 1718 women met inclusion criteria (Figure 1). The majority of women studied were obese (68.4%, 1175), while 31.6% (543) had a BMI <30. In categorizing the obese women, 42.4% (729) had a BMI 30-39.9, 20.0% (344) had a BMI 40-49.9, and 5.9% (102) had a BMI ≥50.
Baseline characteristics of the cohorts are reported in (Table 1). Groups were similar with respect to age, presence of prior cesarean delivery, incidence of repeat and emergency cesarean delivery. Increased BMI was associated with a higher rate of cesarean delivery for dystocia (p<0.002). Tubal sterilization, general anesthesia, and gestational age also varied by BMI cohort.

Figure 1: Flow diagram of participant selection process.
| BMI <30 (n=543) | 30-39.9 (n=729) | 40.0-49.9 (n=344) | ≥50 (n=102) | p value | ||
| Age | 27.7 ± 5.8 | 28.4 ± 5.9 | 28.5 ± 6.0 | 27.8 ± 4.9 | 0.17 | |
| GA at delivery | 37.8 ± 3.3 | 38.4 ± 3.0 | 38.2 ± 2.8 | 38.3 ± 2.6 | 0.03a | |
| Indication for cesarean | Labor dystocia | 14.6% (79) | 18.7% (136) | 23.3% (80) | 29.4% (30) | 0.002 |
| NRFT | 24.0% (130) | 21.1% (154) | 17.4% (60) | 16.7% (17) | 0.002 | |
| Repeat cesarean | 47.4% (257) | 44.8% (391) | 50.9% (175) | 49.0% (50) | 0.18 | |
| Emergency cesarean | 10.1% (55) | 8.6% (63) | 14.7% (21) | 3.9% (4) | 0.07 | |
| Tubal ligation | 15.1% (82) | 19.8% (144) | 22.7% (78) | 21.6% (22) | 0.03 | |
| General anesthesia | 8.8% (38/432) | 5.4% (32/590) | 4.4% (12/276) | 5.2% (4/77) | 0.002 | |
| Data in the form % (number) BMI: Body Mass Index (kg/m2); GA: Gestational Age; NRFT: Non-Reassuring Fetal Tracing p<0.05 considered significant when compared to BMI<30 aOnly BMI <30 is significant |
||||||
Table 1: Baseline characteristics women in each BMI cohort.
The total time in the operating room was significantly longer as BMI increased. Non-obese women had the shortest total median operating time of 95 min, interquartile range 80-109. The median time increased by BMI group: 100 min (84-116), 110 (94.5-128), 126 (112-146) for BMI 30- 39.9, 40-49.9, and ≥50 respectively (Figure 2). Similar trends were noted for incision-to-delivery time and closing time (Table 2). The incision-todelivery interval, increased by 2, 4, and 4 minutes for BMI 30-39.9, 40- 49.9, and BMI ≥50 respectively (p <0.001, Figure 3). In addition to the dose-response seen between BMI and operative times, the time needed to place spinal anesthesia significantly increased with increasing BMI (Table 2, Figure 4). These relationships held consistent when controlling for potential confounding variables including emergency cesarean, repeat cesarean, and general anesthesia as verified with multiple linear regression modeling. In the model, emergency cesarean, cesarean for non-reassuring fetal tracing, repeat cesarean, and additional of a tubal sterilization procedure were noted to alter the total operative time. Removing these subsets of patients did not substantially alter the results.
Blood loss significantly increased with increasing BMI. Average blood loss for BMI <30 was 630 ± 15 ml and increased progressively with BMI: 51 ± 13 ml higher for BMI 30-39.9, 129 ± 19 ml for BMI 40-49.9, and 219 ± 35 ml for BMI ≥50 (p<0.001). Blood loss greater than 1500 ml also increased with increasing BMI (p=0.03). While the blood loss increased by BMI category, the rate of maternal blood transfusion was similar amongst categories. The proportion of patients in which a low transverse uterine incision was employed was significantly lower in BMI ≥50 (51.0% vs. 88.4% in BMI <30; p<0.001). The median length of stay increased with increasing BMI. APGAR scores did not vary between groups.
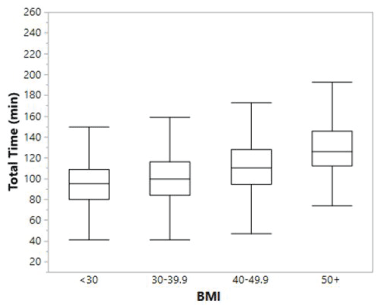
Figure 2: Box plots of total operative time by BMI. Graph depicts the total operative time by BMI cohort (BMI, body mass index kg/m2 ). Box plot shows median time in minutes. Box represents the 25-75 quartiles while the bars are they represent the minimum and maximum values.
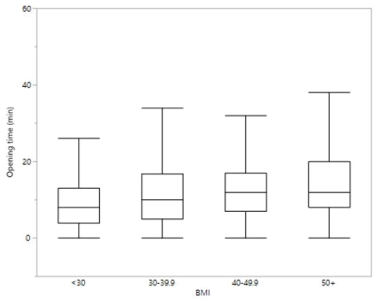
Figure 3: Box plots of incision-to-delivery interval by BMI. Graph depicts the incision-to-delivery interval by BMI cohort (BMI, body mass index kg/m2 ). Box plot shows median time in minutes. Box represents the 25-75 quartiles while the bars are they represent the minimum and maximum values.
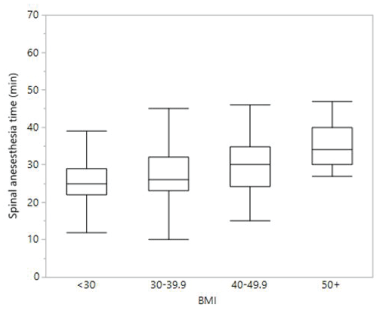
Figure 4: Box plots of spinal anesthesia times by BMI. Graph depicts time for spinal anesthesia by BMI cohort (BMI, body mass index kg/m2 ). Time was measured from the time of anesthesia timeout to skin incision in minutes. Box plot shows median time in minutes. Box represents the 25-75 quartiles while the bars are they represent the minimum and maximum values.
| BMI <30 | 30-39.9 | 40.0-49.9 | ≥50 | p value | |
| Total operative time | 95 (80-109) (n=472) | 100 (84-116)* (n=643) | 110 (94.5-128)* (n=293) | 126 (112 46)* (n=74) | <0.001 |
| Incision-to-delivery interval | 8 (4-13) (n=530) | 10 (5-16.75)* (n=712) | 12 (7-17)* (n=339) | 12 (8-20)* (n=101) | <0.001 |
| Uterine incision-to-delivery interval | 1 (1-2) (n=535) | 2 (1-3) (n=723) | 2 (1-3)* (n=340) | 2 (1-3)* (n=102) | 0.002 |
| Spinal anesthesia time | 25 (22-29) (n=191) | 269 (23-32)* (n=205) | 30 (24.25-34.75)* (n=68) | 34 (30-40)* (n=13) | <0.02 |
| Closing time | 34 (25-42) (n=453) | 35 (26-43) (n=640) | 38 (30-49)* (n=286) | 44 (31-54)* (n=89) | <0.001 |
| Length of stay | 3 (2-3) (n=540) | 3 (2-4)* (n=728) | 3 (2-4)* (n=342) | 3 (2-4)* (n=102) | <0.003 |
| EBL | 630 ± 15 (n=538) | 681 ± 13* (n=723) | 759 ± 19* (n=343) | 849 ± 35* (n=102) | <0.001 |
| EBL>1500mL | 2.8% (15/538) | 4.3%* (31/729) | 5.3%* (18/343) | 8.8%* (9/102) | 0.03 |
| Maternal transfusion | 17.2% (93/541) | 14.0% (102/728) | 13.7% (47/342) | 12.8% (13/102) | 0.33 |
| LTCD | 88.4% (480/543) | 86.2% (627/727) | 84.3% (290/344) | 51.0%* (52/102) | <0.001 |
| Classical uterine incision | 9.2% (50/543) | 11.1%* (81/727) | 14.2%* (49/344) | 43.1%* (44/102) | <0.001 |
| APGAR <7 at 5 min | 4.3% (23/535) | 3.9% (28/725) | 3.2% (11/340) | 1.0% (1/101) | 0.41 |
| BMI: Body Mass Index (kg/m2); EBL: Estimated or quantitative Blood Loss as indicated on operative report (ml); LTCD: Low Transverse Cesarean Delivery; OR: Operating Room Total operating time: Time from room entry to room exit; Closing time: Interval from delivery of the infant to closure of the skin Data are % (n/N), mean ± standard deviation or median (interquartile range); times in minutes Comparisons made with Wilcoxon test for nonparametric continuous variables and Chi-squared for categorical *indicates p<0.05 compared to BMI<30 cohort |
|||||
Table 2: Operative times and other outcomes by BMI category
The current study showed that that total operative time, incision-todelivery interval, closing time, and spinal anesthesia time increased as patient BMI increased. The median surgical time increased by 5 minutes (BMI 30-39.9), 15 min (BMI 40-49.9) and 31 min (BMI ≥50). This trend persisted when controlling for confounding variables including emergency cesarean, repeat cesarean, and general anesthesia. While longer operative time is a risk factor for complications, better preparation and improved surgical strategies targeted to the obese patient have shown mixed results [16,17].
The current study further showed that increased time to place spinal anesthesia with increasing BMI. This is an expected finding as increased BMI makes landmark identification difficult. Ross randomized morbidly obese patients undergoing caesarean to single-shot spinal (SS) or combined spinal-epidural (CSE), finding that CSE was as quick as SS [6]. The authors used a different time metric making time comparisons with our study difficult. Additionally, studies of ultrasound assistance for neuraxial anesthesia have shown mixed results [18].
The rate of cesarean for non-reassuring fetal tracing was surprisingly lower with increasing BMI. This may be explained by technical difficulties with external fetal monitoring in high BMI patients. Our study further showed increased rates of cesarean for arrest of labor, a finding which has been demonstrated in various studies [8].
In agreement with Fyfe et al. [19], our study showed a significant increase in maternal blood loss during cesarean as BMI increased; however, maternal transfusion was not increased. While the average blood loss was increased 219 ml in BMI 50+ compared to BMI<30, the average total blood loss was only 849 ml in BMI ≥50. The similar rate of blood transfusion between groups is likely because the amount of blood loss did not meet the threshold which provoked a blood transfusion.
The number of infants with APGAR score <7 at 5 minutes was similar among BMI cohorts. This observation persisted in a secondary analysis removing patients with emergency cesarean or general anesthesia, factors associated with lower APGAR scores. This finding is comparable to work by Anderson which did not show a relationship between time intervals and low infant APGAR scores or umbilical cord blood values in term, singleton patients undergoing cesarean delivery [20]. Conversely, Conner has shown an increase in neonatal morbidity with increasing BMI [21].
Our data demonstrate a decrease in low transverse uterine incisions and in increase in classical uterine incision with increasing BMI from 9.2% (BMI<30) to 43.1% (BMI ≥50). This likely reflects the increased need for vertical skin incision and the technical difficulties in accessing the lower uterine segment [22,23]. Vertical abdominal incisions have been associated with increased complications. For example, in a study of women with BMI >50, Alanis et al.[24] found a 30% wound complication rate, increased blood loss and a longer hospital stay in patients with vertical skin incisions. Vertical abdominal incisions are often associated with classical uterine entry which is a contraindication to trial of labor after cesarean [25].
Potential limitations in this study include the retrospective nature of the study design and variation in baseline characteristics of the cohorts as possible confounders. While we attempted to control for cofounders through regression analyses, surgeon experience and anesthesiologist experience were not accounted for. Often more experienced providers care for the challenging obese patients and this may bias the time estimates. Because moving times were incorrectly entered in many records, we were unable to assess this outcome. The coding of CSE and epidural anesthetics was problematic due to a lack of distinction between a pre-existing labor epidural and an epidural at time of cesarean. Thus, we limited the analysis to patients undergoing spinal anesthesia. Another limitation was the inability to determine mode of skin incision, cord pH or NICU admission.
The strengths of the study include (1) the large sample size; (2) sufficient power to look at operative times amongst the varying BMI categories; (3) adjustment for possible cofounders with regression analysis; (4) secondary analysis removing cases that were performed emergently, under general anesthesia, or for non-reassuring fetal tracing, to minimize the possibility that such cases might bias the conclusions in the primary analysis; and (5) we tried to account for rare operative complications by removing patients that underwent cesarean hysterectomy
In conclusion, increasing BMI is associated with an increase in total operative time, increased incision-to-delivery interval, increased closing time, increased time for spinal anesthesia, increased incidence of classical uterine incision, and increased blood loss. As the prevalence of obesity continues to increase, these observations will help inform obstetric care we provide to our obese patient.
Download Provisional PDF Here
Aritcle Type: RESEARCH ARTICLE
Citation: Roeckner JT, Sanchez-Ramos L, Kaunitz A (2017) The Impact of Obesity on Operative Time and Blood Loss during Cesarean Delivery. Gynecol Women’s Health Res 1(1): http://dx.doi.org/10.16966/2689-3096.105
Copyright: © 2017 Roeckner JT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access