
Full Text
Vijay Kumar1,* Ashok Kumar2 Sandeep Kumar Singh1 Sanjeev Kumar Tripathi3 nbsp; Dinesh Kumar4 Ragni Singh5 Seema Dwivedi6
1Department of Neurology, SGPGIMS, Lucknow, Uttar Pradesh, India2Department of Medical Genetics, SGPGIMS, Lucknow, Uttar Pradesh, India
3Ass. Prof. Department of Microbiology, GMC, Kannauj, Uttar Pradesh, India
4Department of Chemistry, K.S. Post graduate college, Dr. R.M.L. Avadh University, Faizabad, Uttar Pradesh, India
5Bheem Rao Ambedkar Bihar University, Muzaffarpur, Bihar, India
6Jhunjhunwala Degree College, Dr. R.M.L. Avadh University, Faizabad, Uttar Pradesh, India
*Corresponding author: Vijay Kumar, Department of Neurology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow-226014, India, Tel: +91- 522-2494162; Fax: 91–522–2668017; E-mail: vijaykumarcbt@gmail.com
Purpose: To study the clinical effects of Zinc (Zn) and recent updates on role of zinc in human health, aging process, and immunosenescence.
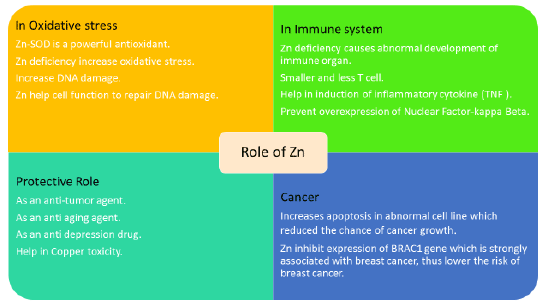
Summary: Zinc has diverse physiological processes, such as growth and development, maintenance of the immune system, and tissue repair. Its deficiency can play a role in the aging process and in the etiology of several age-related chronic illnesses such as atherosclerosis, degenerative diseases of the nervous system, immunosenescence, and cancer. It is also involved in the maintenance of many homeostatic mechanisms, acting as a structural and regulatory catalyst ion for biological activity of many enzymes, proteins, and signal transcription factors, as well as cell proliferation and genome stability. This review will discuss aspects of Zn physiology and its possible beneficial role in the protection. Zn has diversely acts as an anti-oxidant; an organelle stabilizer; an anti-apoptotic agent; and an anti-inflammatory agent. This paper will also review the role of Zn as anti-oxidant has a protective role against oxyradicals and its implications for neurodegenerative and other inflammatory diseases.

Conclusion: Zinc remains a significant public health concern. Deficiency of Zn ions can be leads to inappropriate growth, immunity and other symptoms. A recommended dose of Zn is useful in medication.
Zinc; Brain; Aging; Depression; Deficiency; Oxidative stress
Zinc (Zn) is a critical trace element for humans and animals and is involved in numerous metabolic and signaling pathways within the body. It performing essential roles in various physiological functions such as in mitotic cell division, immune system activity, the synthesis of proteins and nucleic acids, and as a co-factor of enzymes or metalloproteins [1,2]. The human body contains approximately 2 g of Zn, mostly in the testis, muscle, liver, and brain tissues. In the brain, Zn is found at the highest concentrations in the hippocampus, amygdala, cerebral cortex, thalamus, and olfactory cortex. It is one of the prevailing metal ions in the brain and contributes in the regulation of neurogenesis, neuronal migration, and differentiation, thereby shaping cognitive development and maintaining healthy brain function. Zn is available from all food groups, but some important dietary sources of Zn include red meat, poultry, fish, other seafood, legumes, nuts, whole grains, and dairy products [3-12].
The significant role of zinc has been recognized in several biochemical and physiologic functions. It is an abundant and commonly distributed trace element. It has structural, functional, and combined roles in numerous proteins such as hydrolases, transferases, oxido-reductases, ligases, isomerases and lyases. Structurally, zinc is present in different protein domains. Zinc modulates the activity of proteins such as receptors and enzymes that are involved in the regulation of numerous processes, including the synthesis of macromolecules, the regulation of signaling cascades and gene transcription, and transport processes. Zinc acts as a second messenger of intracellular signal transduction has recently been recognized. Zinc is also involved in preserving genomic stability through several actions including regulation of redox homeostasis, DNA repair, synthesis, and methylation. Additionally, zinc can play a role in intercellular signaling in the nervous system and acts as a neurotransmitter [6-8,13-17].
The importance of zinc for humans was recognized in the patients with growth retardation, hepatomegaly, splenomegaly, hypogonadism and severe iron deficiency anemia. Patients with Zn deficiency had severe immune dysfunction because of which they died from opportunistic infections. Zn deficiency causes dysfunction of humoral and cell-mediated immune responses and increases susceptibility to infections. It has also been related to diarrheic diseases with therapeutic benefits being reported in acute diarrhea in children. Zn supplementation produces reduction in diarrhea and pneumonia mortality in children. There have also been reports of benefits with regard to other illnesses such as Wilson’s disease, chronic hepatitis C, leprosy, leishmaniasis, and the common cold [3,6,17-24].
Nutritional zinc deficiency is common in developing countries, and conditioned deficiency of zinc is present in many chronic diseases such as rheumatoid arthritis, diabetes, and cancers, which are associated with chronic inflammation and oxidative stress. Many Studies showed that zinc deficiency increases the concentration of inflammatory cytokines and oxidative stress and induces apoptosis and endothelial cell dysfunction [6,8,23,25-29]. Zinc has critical effect in homeostasis, in immune function, in oxidative stress, in apoptosis, and in aging, and significant disorders of great public health interest are associated with zinc deficiency. In many chronic diseases, including atherosclerosis, several malignancies, neurological disorders, autoimmune diseases, aging, age-related degenerative diseases, and Wilson’s disease, the concurrent zinc deficiency may complicate the clinical features, affect adversely immunological status, increase oxidative stress, and lead to the generation of inflammatory cytokines. In these diseases, oxidative stress and chronic inflammation may play important causative roles. It is therefore important that status of zinc is assessed in any case and zinc deficiency is corrected, since the unique properties of zinc may have significant therapeutic benefits in these diseases. In the present paper, we review the zinc as a multipurpose trace element, its biological role in homeostasis, proliferation and apoptosis and its role in immunity and in chronic diseases, such as cancer, diabetes, depression, Wilson’s disease, Alzheimer’s disease, and other age-related diseases.
Zn is an essential micronutrient for human health in general, and particularly for the elderly. Zinc has critical effect in homeostasis, in immune function, in oxidative stress, in apoptosis, and in aging. In many chronic diseases, including atherosclerosis, several malignancies, neurological disorders, autoimmune diseases, aging, age-related degenerative diseases, and Wilson’s disease, the concurrent zinc deficiency may complicate the clinical features, affect adversely immunological status, increase oxidative stress, and lead to the generation of inflammatory cytokines. In these diseases, oxidative stress and chronic inflammation may play important causative roles. It is hence important that status of zinc is assessed and deficiency is corrected, since the unique properties of zinc may have significant therapeutic benefits in these diseases [3,4,20,22,30].
Under normal condition, homeostatic controls are getting underway to avoid the accumulation of excess Zn or its deficiency. This homeostasis results from the actions of a synchronized regulation effected by different proteins involved in the uptake, excretion and intracellular storage/ trafficking of zinc. These proteins include membranous transporters (ZnT and Zip) and metallothioneins (MT) which control intracellular zinc levels. Interestingly, alterations in ZnT and MT have been recently reported in both aging and AD. The intracellular homeostasis of Zn is regulated by buffer proteins called metallothioneins (MT) which act as storage and transporting proteins of Zn (ZnT and ZIP families) [14- 17,23,31-38].
Zn deficiency in human childhood is known to cause dwarfism, the retardation of mental and physical development, immune dysfunction, and learning disabilities. Recent studies have suggested that secreted Zn2+ plays crucial roles in information processing, synaptic plasticity, learning, and memory. Indeed, Zn2+ has been shown to be essential in the hippocampus for the induction of long-term potentiation, a form of synaptic information storage that has become a well-known paradigm for the mechanisms underlying memory formation. Zn deficiency also adversely affects the immune system, increases oxidative stress, and increases the generation of inflammatory cytokines. However, despite its importance, excess Zn is neurotoxic and has been implicated in neurodegenerative disease. The objective of the present study was to review the results of observational studies of the association of Zn status with its neuroprotective action. Zinc deficiency during pregnancy results in specific impairments in the offspring, which have been observed in animal models but might also be present in humans. Among individuals with Autism Spectrum Disorders (ASD), the incidence rate of zinc deficiency has been reported to be significantly increased compared to age matched healthy control subjects. These low levels of zinc often occur along with copper overload and the Cu/Zn ratio was reported to correlate with the severity of symptoms associated with autism [12,17,27,33,39-47].
Zinc in the Brain
Zinc is abundant in the brain, with particular abundance in the auditory brainstem, olfactory bulb, amygdala, hippocampus, and cortex. It plays a pivotal role in a multitude of cellular processes including neurotransmission, enzymatic activity, gene regulation, and structural maintenance and stabilization of proteins. Due to its widespread function within neurons, intracellular zinc concentrations are tightly regulated. While the majority (80-90%) of the zinc present in the brain is bound to metal-binding proteins, the remaining fraction is packaged within synaptic vesicles of a large sub-population of excitatory neurons. This synaptic or vesicular zinc is released in an activity-dependent manner, and can modulate the activation of several neurotransmitter receptors, including NMDA, AMPA, GABAA and glycine receptors, as well as voltage-dependent ion channels [34,36,48,49].
Zinc is an essential trace element, whose importance to the function of the central nervous system (CNS) is increasingly being appreciated. Alterations in zinc dyshomeostasis has been suggested as a key factor in the development of several neurological ailments. In the CNS, zinc occurs in two forms: the first being tightly bound to proteins and, secondly, the free, cytoplasmic, or extracellular form found in presynaptic vesicles. Under normal conditions, zinc released from synaptic vesicles modulates both ionotropic and metabotropic post-synaptic receptors. While under clinical conditions such as traumatic brain injury, stroke or epilepsy, the excess influx of zinc into neurons has been found to result in neurotoxicity and damage to post synaptic neurons. On the other hand, a growing body of evidence suggests that a deficiency, rather than an excess, of zinc leads to an increased risk for the development of neurological disorders. Indeed, zinc deficiency has been shown to affect neurogenesis and increase neuronal apoptosis, which can lead to learning and memory deficits. Current investigations suggest that Zn deficiency increases the risk of neurodegenerative disorders, affecting neurogenesis and increasing neuronal apoptosis, which can cause a deficiency in learning and memory. This links Zn deficiency to cerebral aging, depression, Parkinson’s disease, and Alzheimer’s disease. It has been postulated that the increased intake of inorganic copper in drinking water can be important in the pathogenesis of Alzheimer’s disease. The elevation of the levels of Zn leads to a decrease of copper levels. A clinical essay indicates more improvement of symptoms in patients suffering from depression supplemented with Zn and antidepressants than in those who were administered a placebo and anti-depressants [15,16,36,50,51].
Zinc deficiency may play a role in increasing the permeability of the blood-brain barrier, or the blood vessel system of the central nervous system that protects the brain from various toxic agents and foreign matter. This permeability is thought to increase drastically during periods of oxidative stress, for example bacterial or viral infection in the body. Zinc, in sufficient quantities, is believed to protect this barrier with its antioxidant properties.
A variety of brain diseases have been associated with lowered zinc levels; these include schizophrenia, alcoholism, Wilson’s disease and Pick’s disease. Zinc has been used as a treatment in resolving some of these diseases, including Wilson’s disease and some types of schizophrenia.
Heightened levels of zinc in some parts of the brain are associated with Alzheimer’s disease. Progression of the disease is correlated with increased zinc levels in various parts of the brain, but also decreased levels in other regions. It is unknown what happens to zinc levels in the brain of Alzheimer’s disease, a redistribution of zinc may be enough to cause the disease to progress. This redistribution refers to the presence of zinc in areas of the brain where it’s not typically found, something which can happen, for instance, with traumatic brain injury.
In a mouse model of Wilson’s disease (toxic milk mutant), hepatic MTs accumulate as a result of decreased protein degradation and this appears to offer some protection from the high hepatic Cu levels. In Wilson’s disease, zinc treatment does not aggravate the patients’ clinical signs and/ or laboratory findings. However, it does improve some clinical symptoms of the patients. Although the administration of zinc has some side effects, none of them is so severe. Thus, Zn acetate is a recommended therapy, for long term management of patients with Wilson’s disease [4,8,40,52,53].
Zinc as Antioxidants
Zinc is an excellent antioxidant. The purpose of an antioxidant is to get rid of free radicals that cause damage to cells in the body by bonding with them and neutralizing them. Zinc is particularly good at countering the damaging effect of high iron. Zinc also targets free radicals that cause inflammation throughout the body. The trace element zinc is linked together in cytosolic defense against reactive oxygen and nitrogen species. Zinc–superoxide dismutase catalyzes the dismutation of superoxide to oxygen and hydrogen peroxide. The latter and other hydroperoxides are subsequently reduced by the selenoenzyme glutathione peroxidase (GPx). Zinc ions may stimulate protective cellular stress-signaling pathways such as the antiapoptotic phosphoinositide-3-kinase/Akt cascade and may stabilize proteins, thereby rendering them less prone to oxidation. One important function of Zn ions in biology is to stabilize proteins. Zn2+ is redox inert in biological systems. Thus, the role of zinc in CuZn– SOD is generally thought to be that of a stabilizing component. Zn2+ is capable of inducing a stress response in terms of [1] the stimulation of MTF-1-dependent transcription, and the activation of stress-responsive signaling cascades such as MAPK and PI3K/Akt. It also stabilization of protein thiols. Zn2+ may also stabilize thiols in the zinc proteins including metallothioneins and zinc-finger transcription factors [54-56].
Zinc has an atheroprotective function because of its anti-inflammatory, anti-oxidant, and other properties [9]. Zinc supplementation would downregulate the inflammatory biomarkers for atherosclerosis in humans. In summary, this study showed that zinc increased antioxidant power and decreased CRP, inflammatory cytokines, adhesion molecules, and oxidative stress markers in elderly subjects after 6 mo of supplementation, and zinc decreased the generation of TNF-α, IL-1β, VCAM-1, and MDA+HAE, as well as NF-κB activation and increased A20 and PPAR-α in THP-1 cells and HAECs, which suggest that zinc may have an atheroprotective effect because of its anti-inflammatory and anti-oxidant function [6,32,57-60].
Oxidative stress is known to be an important contributing factor in many chronic diseases. Zinc acts as an effective anti-inflammatory and anti-oxidant agent. Zinc supplementation may lead to down regulation of the inflammatory cytokines. Zinc protects the cell from oxidation damage by free radicals. This may be due to several factors: acting by stabilizing the cell membrane structure, maintaining an adequate level of MTs (which are free radical scavengers) acting as an essential component of superoxide dismutase (SOD), acting as a protective agent for thiols, and in preventing the interaction between chemical groups with iron to form free radicals, as well as acting as an inhibitor of NADPH oxidase (effective scavenger of radicals). The role of MTs is to buffer cytoplasmic zinc following its influx into the cytoplasm, and so far it seems that temporary cellular zinc storage is the exclusive function of MTs. MTs play a crucial protective role (due to their redox properties) in the presence of radiations, heavy toxic metals, lipid peroxidation, or reactive oxygen species.
Zinc deficiency, after prolonged reduction of intake or excessive uncompensated losses, has been described both in animals and humans and is associated with increased levels of oxidative damage, including increased lipid, protein, and DNA oxidation. Long-term deprivation of Zn renders an organism more susceptible to injury induced by oxidative stress. More specifically, Zn deficiency increases the levels of lipid peroxidation in mitochondrial and microsomal membranes and the osmotic fragility of erythrocyte membranes, while the presence of Zn prevents lipid peroxidation; thus, Zn plays a significant role in protecting the cell from oxidative stress. Recent studies have reported that the metallothionins represent a connection between cellular zinc and the redox state of the cell. Under conditions of high oxidative stress, changes in the cellular redox state result in release of zinc from metallothionin, as a result of sulfide/ disulfide exchange. In cases of stress, antioxidants are absolutely necessary to regulate the reactions that release free radicals [3,6,29,37,48,61-66]. Zinc induces apoptosis in melanoma cells by increasing ROS and this effect may be mediated by the ROS-dependent induction of p53 and FAS/ FAS ligand [60].
The beneficial effects of zinc in the management of infantile diarrhea and acute respiratory infections in children in the developing world, as evidenced by decreased mortality, morbidity, and incidence of infections in patients with sickle cell disease and in the elderly subjects, are due to the important roles of zinc in the cell-mediated immune functions. Zinc is very effective in decreasing the progression of age-related macular degeneration and has prevented blindness in many patients. Another report found that mortality significantly decreased in zinc-treated subjects. Intake of zinc was inversely associated with risk of Crohn’s disease (CD) but not ulcerative colitis (UC) [66].
Zinc (Zn) is an essential transition d-block element. It acts as chelationbased therapeutic agent as well as it has various neuroprotective action. Zinc deficiency, as well as redistribution of zinc in the brain, has been associated with a greater incidence of disease. Children are particularly mentally impacted by zinc deficiency. Insufficient zinc levels in children have been linked to decreased learning ability, apathy, lethargy and mental retardation, according to the National Institutes of Health Library of Medicine. Children who are hyperactive could also be zinc-deficient. Zinc deficiency during pregnancy and lactation has been linked to congenital abnormalities in children’s nervous systems, due to its important role in brain development [42,67-70].
Depression is a common mental disorder associated with functional impairment, significant disability, morbidity and mortality. Clinical studies demonstrate significantly lower serum zinc levels in patients suffering from major depression or unipolar depression than that in non-depressed patients. In some patients, a negative correlation between serum zinc level and severity of depression was found [50,71,72].
Aging is an inevitable process associated with progressive pathological features such as: oxidative stress, altered cell metabolism, damaged of nucleic acid, or deposition of abnormal forms of proteins. Zinc deficiency is usually the result of inadequate zinc dietary intake. Accordingly, zinc has anti-inflammatory properties and a low zinc status is associated with increased susceptibility to infection plus intracellular zinc has been found to play a key role in signaling in immune cells. Then again aging is characterized by the progressive deregulation of immune responses. Therefore, zinc has been suggested as a good factor in providing the remodeling of some age-associated changes and also as leading to healthy ageing through the reduction of inflammation. On the other hand, the supplementation of zinc in aging improves immune function and leads to decreased mortality from infections.
Some of the studies presented above suggest that zinc can be useful not only in it but in combination with other drugs used in treatment. Other important aspects in the context of zinc and treatment of patients are metal chelation drugs, for which the positive effect was particularly emphasized in AD. The weakness of most of these drugs, however, is the side effects caused by the chelation of other important divalent metal ions in the brain. Chelation should thus be used only when the brain zinc level is expected to have neurotoxic effects [26,73].
Recently, zinc-homeostasis regulating proteins such as transporters and MTs have been gaining more prominence in related literature indicating they may be very important players in the pathophysiology of neurodegenerative disorders. Therefore, more studies are needed to fully understand the influence of peripheral zinc deficiency or an overdose on these proteins.
The brain barrier system, i.e., the blood-brain and blood-cerebrospinal fluid barriers, is important for zinc homeostasis in the brain. Zinc is supplied to the brain via both barriers. A large portion of zinc serves as zinc metalloproteins in neurons and glial cells. Approximately 10% of the total zinc in the brain, probably ionic zinc, exists in the synaptic vesicles, and may serve as an endogenous neuromodulator in synaptic neurotransmission. The turnover of zinc in the brain is much slower than in peripheral tissues such as the liver. However, dietary zinc deprivation affects zinc homeostasis in the brain. Vesicular zinc-enriched regions, e.g., the hippocampus, are responsive to dietary zinc deprivation, which causes brain dysfunctions such as learning impairment and olfactory dysfunction. Olfactory recognition is reversibly disturbed by the chelation of zinc released from amygdala neuron terminals. On the other hand, the susceptibility to epileptic seizures, which may decrease vesicular zinc, is also enhanced by zinc deficiency. Therefore, zinc homeostasis in the brain is closely related to neuronal activity. Even in adult animals and probably adult humans, adequate zinc supply is important for brain functions and prevention of neurological diseases [26,73].
The roles of zinc in the developing and adult brain (and other organ systems) are in part due to the fact that zinc is an essential catalytic component of 80 different mammalian enzymes. Many of these enzymes, such as DNA and RNA polymerases, histone deacetylases, and DNA ligases, are clearly needed for normal DNA replication and cellular proliferation. Other zinc dependent enzymes, including metalloproteinases and many dehydrogenases in intermediary metabolism, also play important roles in normal CNS function. Additionally, zinc plays an essential structural role in a family of DNA binding transcription factors known as zinc-finger proteins. Nuclear receptors, such as those that mediate the transcriptional roles of retinoic acid, vitamin D, thyroid hormone, glucocorticoids, and estrogen in the brain, are all zinc-finger proteins. All of these receptors are known to regulate key genes involved in cellular proliferation, brain development, and neurogenesis [34,36,48,49,74].
In addition to the zinc that is bound to enzymes, transcription factors, and other proteins, ∼20% of CNS zinc are in the free form and are associated with pre-synaptic vesicles of glutamatergic neurons. Although neurons containing free zinc are found in many regions of the brain, including the cortex, amygdala, and the olfactory bulb, the neurons of the hippocampus appear to have the highest concentrations of free zinc. Zinc acts as an activity-dependent, endogenous modulator of AMPA-subtype glutamate receptors (AMPARs) that tunes fast excitatory neurotransmission and plasticity in glutamatergic synapses [74].
Zinc is one of the most important trace elements in the organism, with three major biological roles, the catalytic, the structure, and the regulatory one. It is a multifunctional metal compatible with satisfactory growth, health, and well-being. It is essential for the structure and function of various proteins and cellular components and plays an important role in human physiology from its involvement in the proper function of the immune system to its role in cellular growth, cell proliferation, cell apoptosis, as well as in the activity of numerous zinc-binding proteins. However, zinc also plays a key pathophysiological role in major neurological disorders, such as in Alzheimer’s disease, cancer, aging, diabetes, depression, and Wilson’s disease. Significant disorders of great public health interest are associated with zinc deficiency. Many investigators have used zinc supplementation as a powerful therapeutic tool in an attempt to affect the outcome of various diseases. It is therefore important that the status of zinc is assessed and zinc deficiency is corrected in some chronic diseases such as neurological disorders, autoimmune diseases, and aging.
From the foregoing results, it is obvious that zinc homeostasis may play a major role in the initiation and propagation of the pathological features of psychiatric and neurodegenerative disorders. First, since zinc deficiency is prevalent in patients with psychiatric and neurodegenerative disorders, the appropriate preventive measures should be considered especially in the elderly. Conversely, even if the beneficial effects of zinc supplementation were reported either in treatment or in the prevention of depressive or aging symptoms, zinc supplement users should be overly cautious and avoid overdosing [1,2,6,26,27,42,43,45].
Alterations in trace element homeostasis could be involved in the pathology of dementia, and in particular of Alzheimer’s disease (AD). Zinc is a structural or functional component of many proteins, being involved in numerous and relevant physiological functions. Zinc homeostasis is affected in the elderly, and current evidence points to alterations in the cellular and systemic distribution of zinc in AD. Although the association of zinc and other metals with AD pathology remains unclear, therapeutic approaches designed to restore trace element homeostasis are being tested in clinical trials. Not only could zinc supplementation potentially benefit individuals with AD, but zinc supplementation also improves glycemic control in the elderly suffering from diabetes mellitus. Zinc supplementation also suggested playing a possible protective role in the onset of the type 1 diabetes [29]. However, the findings that select genetic polymorphisms may alter an individual’s zinc intake requirements should be taken into consideration when planning zinc supplementation. This review will focus on current knowledge regarding pathological and protective mechanisms involving brain zinc in AD to highlight areas where future research may enable development of new and improved therapies.
Brain aging is marked by structural, chemical, and genetic changes leading to cognitive decline and impaired neural functioning. Further, aging itself is also a risk factor for a number of neurodegenerative disorders, most notably Alzheimer’s disease (AD). Many of the pathological changes associated with aging and aging-related disorders have been attributed in part to increased and unregulated production of reactive oxygen species (ROS) in the brain. ROS are produced as a physiological byproduct of various cellular processes, and are normally detoxified by enzymes and antioxidants to help maintain neuronal homeostasis. However, cellular injury can cause excessive ROS production, triggering a state of oxidative stress that can lead to neuronal cell death. ROS and intracellular zinc are intimately related, as ROS production can lead to oxidation of proteins that normally bind the metal, thereby causing the liberation of zinc in cytoplasmic compartments. Similarly, not only can zinc impair mitochondrial function, leading to excess ROS production, but it can also activate a variety of extra-mitochondrial ROS-generating signaling cascades. As such, numerous accounts of oxidative neuronal injury by ROS-producing sources appear to also require zinc. We suggest that zinc deregulation is a common, perhaps ubiquitous component of injurious oxidative processes in neurons. This review summarizes current findings on zinc dyshomeostasis-driven signaling cascades in oxidative stress and age-related neurodegeneration, with a focus on AD, in order to highlight the critical role of the intracellular liberation of the metal during oxidative neuronal injury [9,28,58,59,75-77].
Zinc is necessary for not only brain development but also brain function. Zinc homeostasis in the brain is tightly regulated by the brain barrier system and is not easily disrupted by dietary zinc deficiency. However, histologically reactive zinc as revealed by Timm’s staining is susceptible to zinc deficiency, suggesting that the pool of Zn++ can be reduced by zinc deficiency. The hippocampus is also susceptible to zinc deficiency in the brain. On the other hand, zinc deficiency causes abnormal glucocorticoid secretion from the adrenal cortex, which is observed prior to the decrease in extracellular zinc concentration in the hippocampus. The hippocampus is enriched with glucocorticoid receptors and hippocampus functions are changed by abnormal glucocorticoid secretion. Zinc deficiency elicits neuropsychological symptoms and affects cognitive performance. It may also aggravate glutamate excitotoxicity in neurological diseases. Abnormal glucocorticoid secretion is associated with these symptoms in zinc deficiency. Furthermore, the decrease in Zn++ pool may cooperate with glucocorticoid action in zinc deficiency. Judging from susceptibility of Zn++ pool in the brain to zinc deficiency, it is possible that the decrease in Zn++ pool in the peripheral tissues triggers abnormal glucocorticoid secretion. To understand the importance of zinc as a signaling factor, this paper analyzes the relationship among the changes in hippocampal functions, abnormal behavior and pathophysiological changes in zinc deficiency, based on the data from experimental animals.
- Prasad AS (1991) Role of zinc in human health. Bol Asoc Med P R 83: 558-560. [Ref.]
- Prasad AS (1995) Zinc: an overview. Nutrition 11: 93-99. [Ref.]
- Chasapis CT, Loutsidou AC, Spiliopoulou CA, Stefanidou ME (2012) Zinc and human health: an update. Arch Toxicol 86: 521-534. [Ref.]
- DiGirolamo AM, Ramirez-Zea M (2009) Role of zinc in maternal and child mental health. Am J Clin Nutr 89: 940S-945S. [Ref.]
- Frassinetti S, Bronzetti G, Caltavuturo L, Cini M, Croce CD (2006) The role of zinc in life: a review. J Environ Pathol Toxicol Oncol 25: 597-610. [Ref.]
- Prasad AS (2009) Zinc: role in immunity, oxidative stress and chronic inflammation. Curr Opin Clin Nutr Metab Care 12: 646-652. [Ref.]
- Rimbach G, Markant A, Pallauf J, Krämer K (1996) [Zinc--update of an essential trace element]. Z Ernahrungswiss 35: 123-142. [Ref.]
- Shankar AH, Prasad AS (1998) Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr 68: 447S-463S. [Ref.]
- Chan S, Gerson B, Subramaniam S (1998) The role of copper, molybdenum, selenium, and zinc in nutrition and health. Clin Lab Med 18: 673-685. [Ref.]
- Dixon LB, Winkleby MA, Radimer KL (2001) Radimer, Dietary intakes and serum nutrients differ between adults from food-insufficient and food-sufficient families: Third National Health and Nutrition Examination Survey, 1988-1994. J Nutr 131: 1232-1246. [Ref.]
- Ervin RB, Kennedy-Stephenson J (2002) Mineral intakes of elderly adult supplement and non-supplement users in the third national health and nutrition examination survey. J Nutr 132: 3422-3427. [Ref.]
- Prasad AS (1983) Clinical, biochemical and nutritional spectrum of zinc deficiency in human subjects: an update. Nutr Rev 41: 197-208. [Ref.]
- Sharif R, Thomas P, Zalewski P, Fenech M (2012) The role of zinc in genomic stability. Mutat Res 733: 111-121. [Ref.]
- Siwek MS, Wróbel A, Dudek D, Nowak G, Zieba A (2005) [The role of zinc in the pathogenesis and treatment of affective disorders]. Psychiatr Pol 39: 899-909. [Ref.]
- Stelmashook EV, Isaev NK, Genrikhs EE, Amelkina GA, Khaspekov LG, et al. (2014) Role of zinc and copper ions in the pathogenetic mechanisms of Alzheimer’s and Parkinson’s diseases. Biochemistry (Mosc) 79: 391-396. [Ref.]
- Tyszka-Czochara M, Grzywacz A, Gdula-Argasińska J, Librowski T, Wiliński B, et al. (2014) The role of zinc in the pathogenesis and treatment of central nervous system (CNS) diseases. Implications of zinc homeostasis for proper CNS function. Acta Pol Pharm 71: 369- 377. [Ref.]
- Andrews RC (1992) An update of the zinc deficiency theory of schizophrenia. Identification of the sex determining system as the site of action of reproductive zinc deficiency. Med Hypotheses 38: 284-291. [Ref.]
- Adamo AM, Zago MP, Mackenzie GG, Aimo L, Keen CL, et al. (2010) The role of zinc in the modulation of neuronal proliferation and apoptosis. Neurotox Res 17: 1-14. [Ref.]
- Aggarwal R, Sentz J, Miller MA (2007) Role of zinc administration in prevention of childhood diarrhea and respiratory illnesses: a metaanalysis. Pediatrics 119: 1120-1130. [Ref.]
- Bajait C, Thawani V (2011) Role of zinc in pediatric diarrhea. Indian J Pharmacol 43: 232-235. [Ref.]
- Basnet S, Mathisen M, Strand TA (2015) Oral zinc and common childhood infections-An update. J Trace Elem Med Biol 31: 163-166. [Ref.]
- Black RE, Fischer Walker C (2012) Role of zinc in child health and survival. Nestle Nutr Inst Workshop Ser 70: 37-42. [Ref.]
- Bonaventura P, Benedetti G, Albarède F, Miossec P (2015) Zinc and its role in immunity and inflammation. Auto immun Rev 14: 277-285. [Ref.]
- Kalita J, Kumar V, Misra UK, Ranjan A, Khan H (2014) A study of oxidative stress, cytokines and glutamate in Wilson disease and their asymptomatic siblings. J Neuroimmunol 274: 141-148. [Ref.]
- Beck FW, Prasad AS, Kaplan J, Fitzgerald JT, Brewer GJ (1997) Changes in cytokine production and T cell subpopulations in experimentally induced zinc-deficient humans. Am J Physiol 272: E1002-E1007. [Ref.]
- Prasad AS (2014) Zinc: an antioxidant and anti-inflammatory agent: role of zinc in degenerative disorders of aging. J Trace Elem Med Biol 28: 364-371. [Ref.]
- Prasad AS, Beck FW, Grabowski SM, Kaplan J, Mathog RH (1997) Zinc deficiency: changes in cytokine production and T-cell subpopulations in patients with head and neck cancer and in noncancer subjects. Proc Assoc Am Physicians 109: 68-77. [Ref.]
- Wintergerst ES, Maggini S, Hornig DH (2007) Hornig, Contribution of selected vitamins and trace elements to immune function. Ann Nutr Metab 51: 301-323. [Ref.]
- Valera P, Zavattari P, Sanna A, Pretti S, Marcello A, et al. (2015) Zinc and Other Metals Deficiencies and Risk of Type 1 Diabetes: An Ecological Study in the High Risk Sardinia Island. PLoS One 10: e0141262. [Ref.]
- Cummings JE, Kovacic JP (2009) The ubiquitous role of zinc in health and disease. J Vet Emerg Crit Care (San Antonio) 19: 215-240. [Ref.]
- Kambe T (2013) [Overview of and update on the physiological functions of mammalian zinc transporters]. Nihon Eiseigaku Zasshi 68: 92-102. [Ref.]
- Karagulova G, Yue Y, Moreyra A, Boutjdir M, Korichneva I (2007) Protective role of intracellular zinc in myocardial ischemia/reperfusion is associated with preservation of protein kinase C isoforms. J Pharmacol Exp Ther 321: 517-525. [Ref.]
- Krebs NF (2013) Update on zinc deficiency and excess in clinical pediatric practice. Ann Nutr Metab 62: 19-29. [Ref.]
- Sekler I, Moran A, Hershfinkel M, Dori A, Margulis A, et al. (2002) Distribution of the zinc transporter ZnT-1 in comparison with chelatable zinc in the mouse brain. J Comp Neurol 447: 201-209. [Ref.]
- Watt NT, Whitehouse IJ, Hooper NM (2010) The role of zinc in Alzheimer’s disease. Int J Alzheimers Dis 2011: 971021. [Ref.]
- Weiss JH, Sensi SL, Koh JY (2000) Zn(2+): a novel ionic mediator of neural injury in brain disease. Trends Pharmacol Sci 21: 395-401. [Ref.]
- Wiseman DA, Sharma S, Black SM (2010) Elevated zinc induces endothelial apoptosis via disruption of glutathione metabolism: role of the ADP translocator. Biometals 23: 19-30. [Ref.]
- Wong CP, Ho E (2012) Zinc and its role in age-related inflammation and immune dysfunction. Mol Nutr Food Res 56: 77-87. [Ref.]
- Black RE (2003) Zinc deficiency, infectious disease and mortality in the developing world. J Nutr 133: 1485S-1489S. [Ref.]
- Hambidge KM, Krebs NF (2007) Zinc deficiency: a special challenge. J Nutr 137: 1101-1105. [Ref.]
- Ploysangam A, Falciglia GA, Brehm BJ (1997) Effect of marginal zinc deficiency on human growth and development. J Trop Pediatr 43: 192- 198. [Ref.]
- Prasad AS (1996) Zinc deficiency in women, infants and children. J Am Coll Nutr 15: 113-120. [Ref.]
- Prasad AS (2000) Effects of zinc deficiency on Th1 and Th2 cytokine shifts. J Infect Dis 182 Suppl 1: S62- S68. [Ref.]
- Prasad AS (2002) Zinc deficiency in patients with sickle cell disease. Am J Clin Nutr 75: 181-182. [Ref.]
- Prasad AS (2003) Zinc deficiency. BMJ 326: 409-410. [Ref.]
- Prasad AS (2004) Zinc deficiency: its characterization and treatment. Met Ions Biol Syst 41: 103-137. [Ref.]
- Solomons NW (1998) Mild human zinc deficiency produces an imbalance between cell-mediated and humoral immunity. Nutr Rev 56: 27-28. [Ref.]
- Pekun TG, Hrynevich SV, Waseem TV, Fedorovich SV (2014) Role of iron, zinc and reduced glutathione in oxidative stress induction by low pH in rat brain synaptosomes. Springerplus 3: 560. [Ref.]
- Singla N, Dhawan DK (2012) Regulatory role of zinc during aluminiuminduced altered carbohydrate metabolism in rat brain. J Neurosci Res 90: 698-705. [Ref.]
- Szewczyk B, Kubera M, Nowak G (2011) The role of zinc in neurodegenerative inflammatory pathways in depression. Prog Neuropsychopharmacol Biol Psychiatry 35: 693-701. [Ref.]
- Smith AP, Lee NM (2007) Role of zinc in ALS. Amyotroph Lateral Scler 8: 131-143. [Ref.]
- Dal Peraro M, Vila AJ, Carloni P, Klein ML (2007) Role of zinc content on the catalytic efficiency of B1 metallo beta-lactamases. J Am Chem Soc 129: 2808-2816. [Ref.]
- Kamer B, Wąsowicz W, Pyziak K, Kamer-Bartosińska A, Gromadzińska J, et al. (2012) Role of selenium and zinc in the pathogenesis of food allergy in infants and young children. Arch Med Sci 8: 1083-1088. [Ref.]
- (2003) Dietitians of, Position of the American Dietetic Association and Dietitians of Canada: Vegetarian diets. J Am Diet Assoc 103: 748-765. [Ref.]
- Johnson AR, Munoz A, Gottlieb JL, Jarrard DF (2007) High dose zinc increases hospital admissions due to genitourinary complications. J Urol 177: 639-643. [Ref.]
- Moncayo R, Kroiss A, Oberwinkler M, Karakolcu F, Starzinger M, et al. (2008) The role of selenium, vitamin C, and zinc in benign thyroid diseases and of selenium in malignant thyroid diseases: Low selenium levels are found in subacute and silent thyroiditis and in papillary and follicular carcinoma. BMC Endocr Disord 8: 2. [Ref.]
- Sreedhar B, Subramaniyan R, Nair KM (2004) A protective role for zinc on intestinal peroxidative damage during oral iron repletion. Biochem Biophys Res Commun 318: 992-997. [Ref.]
- Tuzcu M, Sahin N, Dogukan A, Aslan A, Gencoglu H, et al. (2010) Protective role of zinc picolinate on cisplatin-induced nephrotoxicity in rats. J Ren Nutr 20: 398-407. [Ref.]
- Yoshihara D, Fujiwara N, Ookawara T, Kato S, Sakiyama H, et al. (2009) Protective role of glutathione S-transferase A4 induced in copper/zinc-superoxide dismutase knockout mice. Free Radic Biol Med 47: 559-567. [Ref.]
- Provinciali M, Pierpaoli E, Bartozzi B, Bernardini G (2015) Zinc Induces Apoptosis of Human Melanoma Cells, Increasing Reactive Oxygen Species, p53 and FAS Ligand. Anticancer Res 35: 5309-5316. [Ref.]
- Cruz KJC, de Oliveira ARS, Marreiro DDN (2015) Antioxidant role of zinc in diabetes mellitus. World J Diabetes 6: 333-337. [Ref.]
- Groleau J, Dussault S, Haddad P, Turgeon J, Ménard C, et al. (2010) Essential role of copper-zinc superoxide dismutase for ischemiainduced neovascularization via modulation of bone marrow-derived endothelial progenitor cells. Arterioscler Thromb Vasc Biol 30: 2173- 2181. [Ref.]
- Kang YJ, Zhou Z (2005) Zinc prevention and treatment of alcoholic liver disease. Mol Aspects Med 26: 391-404. [Ref.]
- Sahin K, Sahin N, Kucuk O, Hayirli A, Prasad AS (2009) Role of dietary zinc in heat-stressed poultry: a review. Poult Sci 88: 2176-2183. [Ref.]
- Wages PA, Silbajoris R, Speen A, Brighton L, Henriquez A, et al. (2014) Role of H2 O2 in the oxidative effects of zinc exposure in human airway epithelial cells. Redox Biol 3: 47-55. [Ref.]
- Ananthakrishnan AN, Khalili H, Song M, Higuchi LM, Richter JM, et al. (2015) Zinc intake and risk of Crohn’s disease and ulcerative colitis: a prospective cohort study. Int J Epidemiol 44: 1995-2005. [Ref.]
- Bhutta ZA, Bird SM, Black RE, Brown KH, Gardner JM, et al. (2000) Therapeutic effects of oral zinc in acute and persistent diarrhea in children in developing countries: pooled analysis of randomized controlled trials. Am J Clin Nutr 72: 1516-1522. [Ref.]
- Brooks WA, Santosham M, Naheed A, Goswami D, Wahed MA, et al. (2005) Effect of weekly zinc supplements on incidence of pneumonia and diarrhoea in children younger than 2 years in an urban, lowincome population in Bangladesh: randomised controlled trial. Lancet 366: 999-1004. [Ref.]
- Dodig-Curković K, Dovhanj J, Curković M, Dodig-Radić J, Degmecić D (2009) [The role of zinc in the treatment of hyperactivity disorder in children]. Acta Med Croatica 63: 307-313. [Ref.]
- Leonard MB, Zemel BS, Kawchak DA, Ohene-Frempong K, Stallings VA (1998) Plasma zinc status, growth, and maturation in children with sickle cell disease. J Pediatr 132: 467-471. [Ref.]
- Broun ER, Greist A, Tricot G, Hoffman R (1990) Excessive zinc ingestion. A reversible cause of sideroblastic anemia and bone marrow depression. JAMA 264: 1441-1443. [Ref.]
- Nowak G, Szewczyk B, Pilc A (2005) Zinc and depression. An update. Pharmacol Rep 57: 713-718. [Ref.]
- Fabris N, Mocchegiani E (1995) Zinc, human diseases and aging. Aging (Milano) 7: 77-93. [Ref.]
- Kalappa BI, Anderson CT, Goldberg JM, Lippard SJ, Tzounopoulos T (2015) AMPA receptor inhibition by synaptically released zinc. Proc Natl Acad Sci U S A 112: 15749-15754. [Ref.]
- Lansdown AB, Mirastschijski U, Stubbs N, Scanlon E, Agren M (2007) Zinc in wound healing: theoretical, experimental, and clinical aspects. Wound Repair Regen 15: 2-16. [Ref.]
- Sasaki H, Akamatsu H, Horio T (2000) Protective role of copper, zinc superoxide dismutase against UVB-induced injury of the human keratinocyte cell line HaCaT. J Invest Dermatol 114: 502-507. [Ref.]
- Sidhu P, Garg ML, Dhawan DK (2004) Protective role of zinc in nickel induced hepatotoxicity in rats. Chem Biol Interact 150: 199-209. [Ref.]
Download Provisional PDF Here
Aritcle Type: Review Article
Citation: Kumar V, Kumar A, Singh SK, Tripathi SK, Kumar D, et al. (2016) Zinc Deficiency and Its Effect on the Brain: An Update.Int J Mol Genet and Gene Ther 1(1): doi http://dx.doi.org/10.16966/2471- 4968.105
Copyright: © 2016 Kumar V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
SCI FORSCHEN JOURNALS
All Sci Forschen Journals are Open Access
