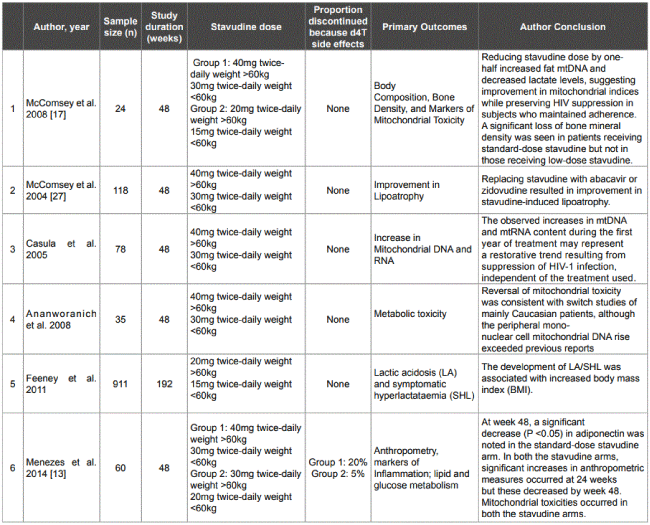
Table 1: Studies that indicated stavudine use at different doses with the outcome.


Deus Buma1* Muhammad Bakari2 Wafaie Fawzi3 Ferdinand Mugusi2
1Department of Pharmacy, Muhimbili National Hospital (MNH), Dar es Salaam, Tanzania*Corresponding author: Deus Buma, Department of Pharmacy, Muhimbili National Hospital, P.O BOX 65000, Dar es Salaam, Tanzania, Tel: +255 787 228282; E-mail: deus.buma@mnh.or.tz
Change of Antiretroviral drugs (ARVs) in the management of HIV/AIDS is not uncommon. Phase-out of stavudine came with challenges in the low-income countries contrary to the developed ones. Much as stavudine was effective similar to other ARVs we reviewed studies that were used to provide evidence for phase out from 1990 to 2016. We noted that stavudine at low dose was effective compared to standard dose in terms of viral load suppression and immune recovery. The associated side effects were significantly alleviated and occurred at longer period when stavudine dose was given at equal to or less than 30mg per day whether given at single or divided doses. The findings provide evidence for its resumption and to be one of the important drugs for patients that cannot tolerate tenofovir, zidovudine or abacavir based combinations.
Phase-out stavudine; initiate-stop-initiate strategy
While we are celebrating more than five years of stavudine phaseout since 2009 as was recommended by World Health Organization (WHO), implementation of this recommendation went with hardship in the developing countries [1,2]. Although incidence of human immunodeficiency virus and acquired immunodeficiency syndrome (HIV/AIDS) is at the decline slope since 2007, the burden still significant high in the developing countries especially sub-Saharan countries. Effort to find effective and cheap antiretroviral therapy (ART) becomes priority to these countries in order to continue providing ART for eligible patients [3]. Developing countries are facing great challenges on budgetary issues [3]. Many developing countries depend on the developed countries to support their budgets include health sector. Health sector has less priority in the developing countries due to other overwhelming priorities as a result their budget depend on support from the developed countries [3]. Developed countries are also facing economic crisis, in this case their support to developing countries are dwindling [4]. Developing countries are inevitably ought to use reasonable cheap regimens in treating their HIV infected patients. Stavudine based regimen will eventually be the first choice before embarking to expensive regimens [3].
We anticipate that stavudine based regimens to be resumed in the few coming years as one of the most important options [5]. This was because its phase-out was based on the studies conducted in the developed countries at higher doses [2]. Stavudine based combinations are relatively cheaper compared to any other available ARV combinations making sustainable HIV treatment programs in the low-income countries feasible [2,3].
We conducted a review of all studies that contained stavudine based combination therapy from 1990 to 2016. These studies are referred to here as “stavudine studies.” We used the following electronic database CINAHL, COCHRANE, EMBASE, Web Science and Medline using the key words; Stavudine, Adults, HIV, Lipodystrophy, neuropathy, immunological, viral load, hyperlipidemia, cardiovascular, standard-dose, reduced-dose and lactic acidosis in various combinations. We included all clinical trials, cohort and retrospective studies for the entire period in which stavudine was used as part of the investigational drugs. We also included studies with follow up duration of greater than six months having a sample size of greater than 20 patients. We also included studies that assessed or reported stavudine related metabolic disorders either major or minor outcome. We excluded all studies that involved stavudine with addition non-antiretroviral drugs or as comparator products.
Stavudine being a thymidine analogue is always combined with purines analogues together with either protease inhibitors (PIs) or non-nucleoside reverse transcriptase inhibitors (NNRTIs) to form a so-called highly active antiretroviral therapy (HAART) [6,7]. The combinations were made in the philosophy of hit-hard, in order to recover immunity for combating opportunist infections as well as ensuring there were successful viral load suppressions [6,8,9] (Table 1). A study conducted by Joly et al. [10] that compared efficacy of Zidovudine (AZT) compared to stavudine (d4T) combinations on virological and immunological recovery indicated that at week 80, 15/85 patients in the AZT arm and 14/85 patients in the d4T arm had reached the primary endpoint, and time to virological failure did not differ between the two arms (P=0.98). In the d4T and in the AZT arms, 67 and 73% of patients, respectively, had HIV-1 RNA levels of <500 copies/ml (P=0.50). The median change from baseline in CD4 cell count was 195 × 106 and 175 × 106 /liter for the d4T- and AZT-containing arms, respectively. The proportions of patients with HIV-1 RNA levels of <50 copies/ml at weeks 8, 16, and 24 were similar in the two arms. The occurrence of serious adverse events was not significantly different between arms. Similar findings were reported by Gallant et al. [11] when stavudine based regimen was compared to tenofovir based combination. The authors indicated that stavudine was comparable to tenofovir in terms of virological outcome in which the proportion of patients with HIV RNA of less than 400 copies/mL at week 48 was 239 (80%) of 299 in patients receiving tenofovir DF and 253 (84%) of 301 in patients receiving stavudine (95% confidence interval, -10.4% to 1.5%).

Table 1: Studies that indicated stavudine use at different doses with the outcome.
Primarily stavudine dose was determined based on body weight [10,12]. Early studies revealed that virological and immunological success was evident if >60kg individual took twice-daily stavudine 40mg while ≤ 60kg patients benefited from 30mg dose [12-15]. However, a study conducted by Ait-Mohand et al. [15] and Hill et al. [16] on viral efficacy and safety reported improved virological efficacy and safety with a reduced dose of stavudine 30mg. In this study, the virological efficacy of 30mg was similar and comparable to stavudine 40mg, but safety was in favor of reduces the dose. Other studies reported a further reduction of stavudine dose to less than 30mg with an increased reduction in stavudine toxicities [16]. A study conducted by McComsey et al. [17] on Effect of Reducing the Dose of Stavudine on Body Composition, Bone Density, and Markers of Mitochondrial Toxicity in HIV-Infected Subjects indicated that further reduction to stavudine 15mg twice daily showed similar efficacy with the standard doses with better safety prognosis [2]. In all aforementioned studies, stavudine doses were taken twice daily [2,14,17,18], other studies have reported once daily used of stavudine simply because its effectiveness depends on intracellular concentration rather than plasma concentration [19-21]. This is the evidence to some extent that even at the reduced stavudine dose [20], still function normally in inhibiting viral replication when combined with other antiretroviral drugs. The evidences that stavudine toxicities increase with increased dose and exposure time [22- 25], disappearance of toxicities with stopping it as well as inhibiting viral replication at lower doses [26,27] even at once-daily dosing [19,20,26], allow stavudine to be the better option in the initiate-stop-initiate strategy when used less than 24 months consecutively [28,29]. A study conducted by Sherwin et al. (2014) on the estimation of intracellular concentration of stavudine triphosphate in children reported that a trough concentration of 13 femtomoles (fmol)/106 cells sufficed to inhibit viral replication, the concentration that can be achieved at stavudine dose of less 30mg per day [2,20,30].
It is not uncommon for HIV to become resistant to many of antiretroviral drugs, particularly when monotherapy were used. Combination therapy alleviates the challenge because when more than two molecules are used, they target at different points in the viral replication cycle [31]. This makes the virus susceptible to drugs with the ultimate reduction in viremia. Among nucleoside reverse transcriptase inhibitors (NRTIs), stavudine is one of the drugs that has high resistant barrier making less likely to cause virologic failure [32], this was also reported by both Matamoros et al. [33], Bradshaw et al. [32] and Nii-Trebi et al. [34] reported that the phenotypic effects of K70G and M184V were similar to those observed with K70E and M184V and those reported for K65R and M184V, affecting multiple NRTIs but with preserved phenotypic susceptibility to zidovudine and stavudine. A study by Lennerstrand et al. [35] on Biochemical Mechanism of HIV-1 Reverse Transcriptase Resistance to Stavudine revealed that, the ATP-dependent d4T resistance effect was most apparent for the codon 69 insertion mutants in an AZT resistance background. The effect of these 69-insertion mutations seems to overlap with the mechanism of resistance to AZT-TP. It is of interest that the corresponding ATPdependent d4T resistance effect was also observed for mutants with AZTspecific resistance mutations at codons 41, 67, 70, and 215. However, the magnitude of resistance observed for d4T was much lower than that for AZT. Similar findings were reported by Gallant et al. [11] when stavudine was compared with tenofovir based regimens. In such report, it was shown that virologic failure was associated most frequently with efavirenz and lamivudine resistance. Through 144 weeks, the K65R mutation emerged in 8 and 2 patients in the tenofovir DF and stavudine groups, respectively (P=0.06).
Stavudine has been implicated as the causal of changes in body fat distribution (lipodystrophy), although is a characteristic of all NRTIs [36-40]. A study conducted by van Oosterhout et al. [23] reported lipodystrophy was one of the distressing adverse effects to the extent that patient declined attending clinic as well as using ART, great condemnation was put to thymidine containing regimens [41]. In this study, stavudine was used at the dose of 30mg regardless of body weight [23,37]. A study by Benn et al. [37] and McComsey et al. [27] reported improvement of cheek and limb fat at week 48 after switching away from thymidine analogy for a patient who had severe baseline lipoatrophy [39,40]. However, studies have reported more lipodystrophy and/or other side effects at higher stavudine doses compared to lower doses [15,17,22,24,26,29,42].
Peripheral neuropathy is a condition resulting from damage to peripheral nerves. Although can also affect another part of the body, often causes weakness, numbness and pain in the hands and feet. Peripheral neuropathy can result from traumatic injuries, inherited causes, exposure to toxins, infections and metabolic problems [43]. People with peripheral neuropathy generally describe the pain as stabbing, burning or tingling. In many cases, symptoms improve, especially if caused by a treatable condition and/or retraction of the causative agent. Many medicines include stavudine can cause peripheral neuropathy [23,43]. A study conducted by van Oosterhout et al. (2012) on Stavudine Toxicity in Adult reported 19.8% of 253 participants in the second year that developed peripheral neuropathy [23]. Although stavudine is implicated in causing peripheral neuropathy at a higher dose (>60mg/day) and prolonged exposure more than two years, such effects are less reported at lower (<40mg/day) doses and less than two years of exposure [13,16,17,29,30,42-44].
Changes in the metabolic state may lead to various conditions including an increase in lipid levels. Increase in lipid levels may result into the occurrence of cardiovascular diseases due to fat deposition in the blood vessels [13,45,46]. Many studies have reported the effects of stavudine on the increase of lipids and triglycerides and other side effects at the standard dose but not at the lower doses [13,17,23]. A study conducted by van Oosterhout et al. [23] on Stavudine Toxicity in Adult reported an increase of lipid and triglyceride at stavudine standard doses but less such increase at low doses [14].
Lactic acidosis is a medical condition characterized by the buildup of lactate (especially L-lactate) in the body, which results in an excessively low pH. It is a subtype of metabolic acidosis, where excessive acid is due to a problem with the body’s metabolism. Apart from other causes, stavudine can influence the accumulation of lactate [47]. A study conducted by Gerard et al. [48] reported that the incidence of lactic acidosis was higher in the patients treated with stavudine based regimens compared to non-stavudine based regimens. Studies by Ait-Mohamed et al. [15] and McComsey et al. [17] reported a decrease in lactate levels at low doses compared to higher doses.
Stavudine being one of the important drugs in the HIV era, we consider to having it in future. Stavudine related side effects that are dose and time-dependent their effects are significantly alleviated when the drug was stopped [27]. Therefore the side effects can be managed by administering lower doses in combination with other drugs, stopping the drug for a while can be an option. The initiate-stop-initiate strategy might be the corner stone for those patients that can experience earlier side effects. Much as stavudine phase-out was based on its side effects at higher doses, less attention was made to studies that showed its effectiveness at lower doses before the whistle for phase-out was blown. Our study provides evidence to re-consider stavudine as one of the ART combinations. Resumption of stavudine will benefit patients that cannot tolerate tenofovir and zidovudine based regimens, in this case, treatment options will be increased. Developing countries with limited resources will be able to treat many patients in this era of test-and-treat campaign for those individuals who are infected with HIV.
There were no potential conflicts of interest disclosed.
Download Provisional PDF Here
Article Type: Review Article
Citation: Buma D, Bakari M, Fawzi W, Mugusi F (2017) Complete Phase-Out Stavudine Based Regimens in the Treatment of HIV-Infection: is it True That Stavudine Hasn’t Any Benefit Today? A Review. J Epidemiol Public Health Rev 2(5): doi http://dx.doi.org/10.16966/2471-8211.152
Copyright: © 2017 Buma D et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access