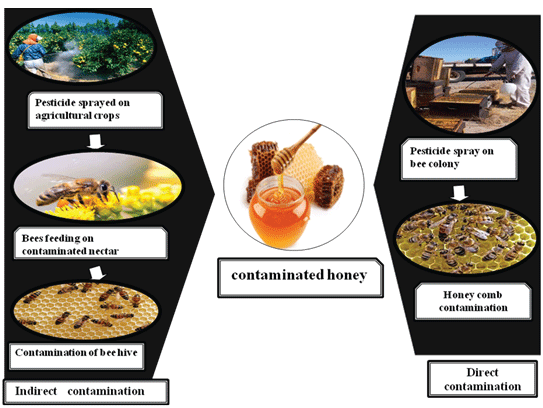
Figure 1: Ways of contamination of honey


Sehrish Nazir* Nazia Rafique Karam Ahad
Ecotoxicology Research Institute, National Agriculture Research Centre, Islamabad, Pakistan*Corresponding author: Nazir S, Ecotoxicology Research Institute, National Agriculture Research Centre, Islamabad, Pakistan, Tel: +992-5190733226; Fax: +992-51-9255034; E-mail: sehrishh50@gmail.com
Honey is under great experimentation now-a-days due to presence of pesticide residues in it. Consumers of the honey products are at stake because pesticide residues are hazardous to health. There is a clear evidence of adverse health related issues due to the pesticides being sprayed. To ensure the food safety for the consumers in compliance with the EC and Codex Alimentarius regulations, highly sensitive, selective and reliable analytical methods are required. Honey is a complex matrix, so the choice of extraction procedure should be appropriate one. This work is focused upon the review of extraction methods and instrumentation used for the analysis of pesticide residues in honey.
Honey; Extraction methods; Multiple pesticide residues; Chromatographic; Spectroscopic techniques
Honey is considered as pure natural product and it is being used for curing diseases like asthama, gastrointestinal disorders, burns and infectious wounds [1]. As it is of primary importance for human health, one should be conscious of its purity. With the growing world population, demand for food has increased enormously; agriculture sector has also boosted up to meet with the requirement. To enhance the production of the food and combat with the agents harmful for the plants different strategies and techniques have been used amongst them fertilizers and pesticides are of greater concern. As far as protection of the crops from the ravage of insects is concerned, a huge number of pesticides are available in market and are being used frequently [1]. Especially, in developing countries trend of using pesticides has increased to greater extent to multiply production in order to meet up with the needs and reduce losses due to pest infestation [2]. Recently, it has become of greater concern as it is a direct challenge for food safety standards.
There are two major sources of contamination in honey, first when honeybees take contaminated nectar along with them and contaminate the colony, ultimately transferring it further to food chain [3,4]. In direct contamination, certain chemical therapeutic agents like coumaphos, fluvalinate, flumethrin, amitraz are applied on hive to combat against larvae diseases and mites [5].The principle ways of pesticides contamination in honey are shown in Figure 1.

Figure 1: Ways of contamination of honey
Since 1998 scientists from Spain [6,7], Greec [8,9], Purtagal [10],Brazil [11], France [12], Italy [13], Serbia [14], Iran [15], Egypt [16], Belgium [17], USA [18], China [19], Argentina [4], Poland [20] have determined pesticide residues in honey and reported the presence of enormous levels of residues above MRL’s. Different national organizations have established MRL’s for honey, but there is no homogeneity among the MRL’s set by different countries which is a big hurdle for analyst but most importantly for traders. According to the European Union (EU) regulations, honey as a natural product must be free of chemicals; maximum concentration of pesticides in honey could be 10 ng/g (http://europa.eu/legislation summaries/food safety/). Codex ailmentarius have’nt established MRL’s for any pesticide in honey till now.
Analytical methodology for determination of residue of pesticides from honey includes extraction, enrichment, and isolation of pesticides from matrix greatly influences the reliability and precision of the analysis. Researcher have reported different extraction methods globally such as, supercritical [17], supercritical fluid extraction [18], solid-phase extraction [21,22], solid phase micro extraction [13,21] stir bar sorptive extraction [23] single drop microextraction, dispersive liquid liquid microextraction [15,9] ultrasound-assisted emulsification microextraction, coacervative microextraction technique (alternative to organic solvents microextraction) [4] QuEChERS [16,24]. Gel permeation and adsorption chromatography are also used to minimize the matrix effect in pesticides analysis from honey [11]. The details of these methods are available in Table 1.
| Pesticides | Extraction/Clean up technique | Solvent system | Recovery (%) | Reference |
| Organochlorine, Pyrethroids | LLE | ethylacetate, ethylacetate : water (80:20 v/v) | 68-126 | [23,25,26] |
| Chlorpyrifos, Cypermethrin, λ-Cyhalothrin, Deltamethrin | LLE-LTP (low temperature purification) | acetonitrile + ethyl acetate | 84-100 | Pinho et al. [10] |
| Amidosulfuron atrazine carbofuran, chlorotoluron Diethofencarb, diethofencarb Dimethoate Fipronil Imidacloprid Isoxaflutole Linuron Methiocarb Methiocarb sulfoxide Metosulam PirimicarbRimsulfuron Simazine Hydroxyterbuthylazine | OCLLE (on column liquid liquid extraction , using Chem Elute cartridge) | 2.5ml acetonitrile + 1.25ml water | 71-90 | Pirard et al [17] |
| Atrazine, simazine, Boscalid, imidacloprid, tau- fluvalinate, thiacloprid | Ultrasonic LLE Extraction time 20min | benzene+water(1:1),Ethyl acetate | 69-92 | [27,28] |
| Organochlorine, organophosphorus, organonitrogen and pyrethroid) | SFE with SFX-220 extraction system+syphonated C02 cylinder and cleanup with SPE Extraction time 20min | for SPE clean up preonditioned with 5 ml (1:1) ethyl acetate/n-hexane two portions of 5mL each, methylene chloride/n- hexane (80:20,v/v) and n-hexane/acetone (60:40, v/v). | 75-94 | Rissato, et al. [18] |
| Organochlorines, Organophosphorus, Carbamates Fenoxycarb Phenthoate Fonofos Diazinon Phosalone Pyrazophos Chlorpyrifos methyl, Profenofos Pirimiphos ethylTemephos Bromophos ethyl Chlorpyrifos ethyl | SPE with C18,HLB,RAM- MISPE, MISPE, Florisil,C18,RP-C18 | preconditioned with 10ml methanol and 10ml water eluted with ethyl acetate 10 ml, methanol 4 ml, dichloromethane 1ml. | 80-120 | [10,21,22,8] |
Table 1: Conventional extraction methodologies for multi-pesticides residues analysis
For the identification and quantification of low levels (ppb or sub ppb) of pesticides residues more sensitive and selective chromatographic methods (GC-ECD, GC-MS, GC-MS/MS, GC-NPD, LC-APCI-MS , LC-MS, LCMS/MS, GC*GC-TOFMS) [23,17,20,29-31] are in use now a day’s (Table 2). This work would be focusing upon the critical review of conventional and advanced analytical techniques used for pesticide residue determination in honey with emphasis upon the extraction procedures.
| Pesticides | Extraction technique (Extraction time, fiber type) | Solvent system | Recovery | reference |
| Chlopyriphos-methyl, Diazinon Fonofos Phenthoate Phosalone Pirimiphos ethyl,Phorate Fenitrothion Malathion Parathion Quinalphos | SBSE, PMDS/PVA | methanol,Acetonitrile | 40-64%, 81-124% | [23,19] |
| Demeton-S-methyl , a-HCH , Lindane, Vinclozolin, Aldrin Chlorpyrifos Malathion Parathion, Chlorfenvinphos (Z isomer) Endosulfan A ,4,4’-DDE, Captan ,2,4’-TDE , Endrin ,Ethion 4,4’-DDT Acrinathrin Methoxychlor ,Tetradifon Phosalone, Fluvalinate 1 Fluvalinate 2, chlorfenvinfos, Diazinon, ethion, pirimiphos methyl, terbufos, (Amitraz, bromopropylate 2,4-dimethylanilne coumaphos, cymiazole fluvalinate), Dichlorvos, phorate, dimethoate, diazinon, methyl parathion, ethion, fenitrothion, malathion, triazophos, fenthion, chlorpyrifos, Fenoxycarb Phenthoate, Fonofos, Diazinon, Phosalone, Pyrazophos Chlorpyrifos methyl, Profenofos, Pirimiphos ethyl, Temephos, Bromophos ethyl, Chlorpyrifos ethyl, Chlopyriphos methyl, Diazinon, Fonofos, Phenthoate, Phosalone, Pirimiphos ethyl | SPME polydimethylsiloxane fibers | 5-127% | [6,19,13,21,23] | |
| CME-UABE | 60 ul n-hexane | ≥ 90% | Fontana et al. [4] | |
| Amitraz, Organochlorine compounds, neonicotinoids | DLLME | Disperser solvent acetonitrile, acetone, Extraction solvent CCl4 ,chloroform | 69.2-119% | [15,20,9,14, |
| Five triazole pesticides (penconazole, hexaconazole, diniconazole, tebuconazole, and difenoconazole) | Elevated temperature -DLLME/ET-DLLME PH 4-8 Temperature 75°C Centrifugation time 5mint Speed 4000 rpm | Extraction solvent:1,2-DBE =130 ul Disperser solvent: DMF = 1.5 ml | 97-100% | [30] |
| Amitraz | HF-LPME | Extracting solvent 1-undecanol | 90-98% | Yamini, Faraji et al. [32] |
| Diazinon, lindane, chlorpyrifos methyl, α-endosulfan, β-endosulfan, 4,4-DDT | HS-SDME | 74-102% BQL for chlorpyrifos and β-endosulfan | Amvrazi, et al. [33] | |
| D-SDME | n.g | |||
| Triazoles and Triazines | AALLME PH 4-8, extraction number 3 centrifugation speed and time 4000 rpm and 5 min | extraction solvent 1,2-DBE (70 ul) | 61-95% | Farajzadeh, Mogaddam et al. [30] |
| OCP’s OP’s Pyrethroids, neonicotinoids and certain organonitrogen, Trichlorfon, Trifluralin, Hexachlorobenzene, Lindane, Chlorpyrifos methyl, Chlorothalonil, Kresoxim methyl, Heptachlor, Malathion, Chlorpyrifos ethyl, Bromophos methyl, Fipronil, Heptachlor epoxide, Endosulfan alpha, 4,4-DDE, Dieldrin, Endrin, I Endosulfan beta, Endrin II, Trifloxystrobin, Endosulfan sulfate, Endosulfan sulfate, Tetradifon, Lambda- cyhalothrin | dSPE QuEChERS kits | Acetonitrile, Acetonitrile acedified with acetic acid | 70-120% | [24,34,29,14,31,21] |
| Organophosphorus. carbamate, amide | MSPD | 5 ml n-hexane-ethyl acetate mixture (90 + 10 v/v) | >80% for oganophosphorus 60% for carbamate & amide | Sanchez-Brunete, et al. [35] |
Table 2: Advanced extraction techniques for analysis of multi-pesticides residues
The selection of method for pesticides extraction from honey should be cautious one, as honey is complex matrix. Extraction with organic solvents, followed by some form of purification to eliminate the coextracted fat is a sequence usually applied. Sample preparation for honey includes, homogenization, extraction, pre-concentration, cleanup, and final concentration prior to analysis. An efficient sample preparation technique should be easy, rapid and efficient in sample cleanup and with operational cost. Different sample preparation procedures were developed and applied during last decades, amongst them are 1) LLE/SE 2) LLME 3) SFE 4) SPE 5) MSPD 6) SPME 7) SBSE 8) QuEChERS 9) SBSE etc.
(i) Liquid liquid extraction (LLE):Among the classical techniques, one is solvent extraction termed as liquid liquid extraction (LLE) in which different water-immiscible solvents and solvent mixtures are used for extraction depending upon the polarity of the respective pesticides. The method is based on the partition of analytes between the aqueous and organic phase. The polarity of the solvent is a trade-off between acceptable recovery and good measurements. Blasco, Fernández et al. [23] used this tehnique to extract organochlorines with three different solvents n-hexane, ethyl acetate and light petroleum and found ethyl acetate was the best solvent for extraction of these compounds with mean recovery 68-126% [Table 1].
The LLE efficiency was improved when single extraction solvent was replaced with solvent mixture (ethyl acetate to ethyl acetate and water mixture (5:1). Louca Christodoulou, Kanari et al. [25] used this approach and extracted 13 organochlorines, 8 pyrethroids and 146 pesticides belonging to different groups (organophosphorus, carbamates, triazoles, amides, neonicotinoids, strobilurines, phenylureas, bendimidazoles and others) and found recoveries in the range of 70- 120%, 73-111% and 71-101% respectively.
The efficiency of LLE is further enhanced and matrix effect is reduced when it was coupled with low temperature purification. The aqueous phase, containing the sample components in frozen form while pesticides extracted with the organic phase. de Pinho, Neves et al. [11] applied this modified method for the extraction of OPPs and pyrethroids pesticides from honey and found good recoveries with minimal matrix effect [11]. This technique has certain drawbacks as it consumes huge quantities of solvent, emulsion formation, time consuming, laborious and large amounts of waste is generated which is not environment friendly [27,5].
(ii) Ultrasonic solvent extraction: This technique has been employed to reduce the consumption of solvent, lower the time, and to enhance the recovery. Alehagen, et al. [28] used sonication technique for the extraction of Boscalid, imidacloprid, tau-fluvalinate and thiacloprid from honey by using ethyl acetate as extractant solvent with recovery within acceptable range (69.4-91.8%) [28].
(iii) Supercritical fluid extraction (SFE ) involves the unique property of supercritical fluids for the extraction of the analyte. It has gained potential importance over conventional SE method because of being fast, using minimum solvent amount, little sample volume and selective extraction can be done through it. Commonly used supercritical fluid is SC CO2 ,which is a good replacement for halogenated solvent because of CO2 being less toxic [36,18] performed SFE with SFX fibers and sulfonated CO2 cylinder to extract organochlorines, organophosphorus, organonitrogen and pyrethroids while cleanup was done with SPE and found recoveries around 75-94% [18]. Among the limitations, it is not economical and pesticides dissolved in water can not be treated through this procedure due to low solubility of CO2 in water [5].
(iv) SPE (solid phase extraction) has advantage over SE for less solvent consumption, its robust, rapid and comparatively simple method. Blasco et al., [10] performed SPE by using C18 as sorbent for the extraction of 9 organochlorines, 5 carbamates, and 28 organophosphorus and found recoveries within acceptable range (73-95%) for all the selected pesticides except Dimethoate with recovery <50% [10]. The extraction efficiency of SPE for Dimethoate and other OP’s was enhanced when sorbent was changed to RAM-MISPE, HLB, MISPE, Florisil, C18, RP-C18, PSA, GCB are also used as sorbent for different classes of pesticides by different scientists globally in order to minimize the matrix effect lack of selectivity, large sample volumes and cartridge material as made of plastic which can absorb the analyte, resulting in interference problems with the analyte [4,5].
(i) SPME (Solid phase micro extraction) is a popular technique as it reduces preparatory steps by doing extraction and pre-concentration simultaneously. In this technique, fused silica coated fiber is dipped in sample and then analytes are either directly desorbed from the fiber into the injection port of a gas chromatograph or by using a polar organic solvent, such as methanol or acetonitrile. The sensitivity and selectivity of extraction by SPME is dependent on type of SPME fiber type. Comparative assessment of extraction efficiency of SPME fibers in terms of extraction time and mean percent recovery was presented in Figure 2. Sol-gel crown ether fiber had greater recovery and least extraction time for analysis of multiple pesticides from honey. this technique eliminates the problems associated with SPE, as described previously, it retains following advantages: (i) it’s a solvent free system, (ii) largely reduced extraction time, (iii) provides good results over a wide range of analyte concentrations, and (vi) can be easily automated. However it shows input sample limitations and relatively high LOD’s. It is relatively expensive technique reason is the fibers are costly [23].
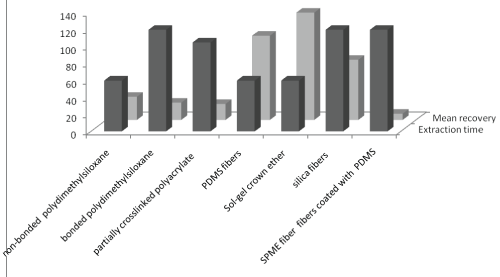
Figure 2: Extraction efficiency of SPME fibers
(ii) SBSE (Stir-bar sorptive extraction) a relatively new technique, it is similar to SPME. In SBSE sample is stirred with a stir bar coated with PDMS fibre, and extraction of analyte is done by partitioning between the polymer and aqueous phase based on the distribution constant. After extraction the solutes are injected into the analytical system either through liquid desorption (LD) or thermal desorption (TD). SBSE was used for extraction of organophosphates from honey samples by using methanol as extaction solvent with PDMS fibers and recoveries obtained were 40-64% [23].The extraction efficiency of SBSE for OPP’s and OCP’s was enhanced with polyvinyl coating to PDMS and acetonitrile as extraction solvent [19]. SBSE uses larger solvent volumes and surface area coating (50-200 times) and brings higher sensitivity and better reproducibility. It is more accurate and sensitive technique and matrix effect of honey in quantification is lower in it as compared to SPME [23].
(iii) MSPD (Matrix solid-phase dispersion) is a novel extraction and clean up technique purposefully developed to avoid the issues encountered in SE and SPE. It requires less solvent and time as compared to conventional methods. The polar compounds and pigments are retained on adsorbent and analyzed directly in this technique. The extraction and cleanup steps are performed in a single step by utilizing small amount of organic solvent. It eradicates the diluting step for solid or semi-solid samples [35,5].
(iv) CME-UABE (Coacervative miroextraction ultrasound-assisted back-extraction technique) was introduced in 2010 by AR Fontana [4] and coworkers for OPP’s extraction. This extraction/pre-concentration technique is supported on micellar organized medium based on non-ionic surfactants, analyte is back extracted with hexane to make compatible with GC, because surfactants are highly viscous and have low volatility [4]. Its economical, easy to operate and environment friendly, uses surfactants thus lowers the consumption of organic solvents [4].
(V) LPME/LLME: liquid phase microextraction/liquid-liquid microextraction technique similar to the LLE but with reduced solvent volume, it has certain types like single drop microextraction (SDME), dispersive liquid liquid microextraction (DLLME) [14], air-assisted liquid-liquid microextraction (AALLME) [30], hollow fiber protected liquid phase microextraction (HF-LPME) [32].
(Va) DLLME (Dispersive liquid-liquid microextraction) is an advanced technique in which water-insoluble extracting solvent is dissolved in a water-soluble dispersive solvent such as acetone. The obtained mixture is then injected into the centrifuge tube containing water sample. Extraction solvent being insoluble in water creates emulsion, increasing contact area between the phases and establishes extraction equilibrium quickly as compared to conventional liquid-liquid extraction. After centrifugation a particular amount of extraction solvent is taken from the tube and injected into the instrument [15,20,9] proposed dispersive liquid-liquid microextraction protocol for the determination of residues of 15 organochlorine pesticides in honey and compared it with SPE, SFE, QuEChERS, SPME LLE, LLE-LTP and results proved that DLLME is best technique with respect to sensitivity , time and low operational cost [9]. It is a simple and economical method with high extraction efficiency while taking short time for extraction but it uses large volume of solvent especially halogenated solvent (highly toxic nature that is difficult to handle in the laboratory ) which is major drawback of this technique [9,30].
(Vb) HF-LPME (Hollow fiber liquid phase microextraction) is a micro-extraction technique in which hollow fibers containing the extractants inside the lumen are used. Thus sample is vigorously stirred without loss of extractant. HF-LPME is the most robust and reliable alternative for typical LPME. There are two modes of this system, twophase HF-LPME and three-phase HF-LPME. Generally two-phase HFLPME is applied when the analytes have high solubility in non-polar organic solvents and three-phase is applied in case of basic/acidic analytes containing ionizable functionalities. Selection of extractant solvent is crucial step in this technique. It must have following characteristics (i) it should me immiscible with water to reduce the loses, (ii) it must have compatibility with the fiber and should be immobilized easily in the pores of hollow fiber; (iii) it must show good chromatographic behavior. Its fibers are not very costly so it can be widely used as far as economical point of view is concerned. It is simple to use, efficient and excellent for cleanup and involves less solvent consumption. Simple LPME provides lesser recoveries as compared to HF-LMPE [32].
(Vc) AALLME (Air assisted liquid-liquid microextraction) a relatively novel technique, introduced by Farajzadeh et al in [30]. A small amount of extractant solvent is added into the aqueous phase containing analyte. This mixture is taken into a syringe and pushed out into a tube for predetermined cycles in order to produce a cloudy mixture. This mixture contains the extractant dispersed as minute droplets into the aqueous phase. Phase separation is done by centrifugating the tube containing mixture and next step is proceeded with the sediment phase. Farajzadeh et al [30] compared the proposed method’s results with previously reported protocols for the determination of the same pesticides e.g. SPME, SPE-DLLME, SBSEDLLME and concluded that AALLME has good repeatability than others. It’s a disperser solvent free technique hence completely rapid. It is more or less similar to DDLME, having advantage of using smaller amounts of solvent. Solvent chosen must have different and higher density than sample, good gas chromatography behavior, less soluble in water and most importantly must have high extraction efficiency with analyte [37,30].
(vi) SDME (single drop micro extraction) is a solvent miniaturized microextraction technique. This technique employs single microdroplet of organic solvent suspended at the tip of the microsyringe needle and sample solution either can be directly pre-concentrated through D-SDME (direct SDME) or through headspace SDME(HS-SDME). It’s convenient to use and reduces cost in comparison with SPME and HF-LPME, highly sensitive and eliminates matrix effect greatly [33].
(vii) QuEChERS: This method is acronym for quick, easy, cheap,effective, rugged and safe. it was first introduced in 2003 and was further modified during recent years. Now-a-days it has become most frequently used technique for the determination of multi-residue pesticides. Its principle is based upon the dispersive solid phase extraction. The analyte is extracted with an organic solvent or mixture of oganic solvents, water is removed by salting out, afterwards the extract is cleaned by passing through SPE sorbent kit rather than SPE column, and finally the extract is analyzed through a suitable technique. Blasco et al., [21] extracted honey samples with QuEChERS and compared its extraction efficiency with SPE, SPME, and PLE. Results indicate that QuEChERS gave the highest recoveries in comparison with other techniques [21]. A number of other researchers also analyzed different pesticides by using this method and found it reliable method with adequate clean up, satisfactory recoveries and repeatability [16,29,24,31,34]. It is considered as an advanced technique, among characteristics are, (i) less time consumption,(ii) reduced waste generated, (iii) minimized matrix interference, (iv) low financial cost (v) it has also introduced ease in operation thus minimizing the potential source of error [31].
A number of extraction & clean up protocols have been developed by analysts so as to reach the maximum easiness and robustness and above all economical. To date quick, robust and effective extraction and cleanup methods have been proposed and successfully practiced as shown by the results given.
Another important step in analysis of pesticides residue from honey after extraction and cleanup is separation of selected analytes through chromatography. Gas chromatography as well as liquid chromatography is being used as separation technique coupled with some detector. Ideal detectors used for the detection and quantification of pesticide residues would respond only to target analyte, while other coextracted elements remain transparent. Table 3 summarizes all available analytical methods with corresponding references.
| INSTRUMENT | ANALYTE | LOD | reference |
| GC-ECD | Organochlorines, Organophosphorus, pyrethoids, Trichlorfon Trifluralin | 0.1-30 ug/kg | [6,8,18,16,11,9,31,24] |
| GC-μECD | Organochlorines, Organophosphorus | 0.07-19 ug/kg | Amvrazi, et al. [33] |
| GC-NPD | Organophosphorus, Pyrethroids, certain organonitrogen and triazole pesticides | 0.05-0.21 ng /g | [30,33] |
| Capillary GC-NPD | Ops, carbamate, amides | 6-15ug/kg | Sanchez-Brunete, et al. [35] |
| GC-FPD | Dichlorvos, phorate, dimethoate, diazinon, methyl parathion, ethion, fenitrothion, malathion, triazophos, fenthion, chlorpyrifos, Quinalphos, ethoprophos, terbufos and fenamiphos | 4-80 ng/kg | [19,38,22] |
| GC-FID | Amitraz, Triazoles and Triazines | 1.2-5 ug/kg | [15,30,32] |
| GC-MS | Organochlorines; Fenitrothion, chlorpyrifos, parathion, methidathion | 0.3-2.5 ug/kg | [10,26,4] |
| GC-IT/MS | chlorfenvinfos, , Diazinon, ethion, pirimiphos methyl, terbufos | 0.2-3 ug/kg | [13,20] |
| GC*GC-TOFMS | Alachlor, Bifenthrin, Diazinon, Organochlorines, Pyriproxyfen, Quinalphos, Vinclozoline | 2.5-16 ng/g | (Barganska, Olkowska et al. [29] |
| GC-MS (quadropole) | Amitraz, bromopropylate 2,4-dimethylanilne coumaphos, cymiazole fluvalinate | 0.3-10 ug/kg | |
| LC-APCI-MS | Chlorpyrifos-methyl, Diazinon, Fonofos, Phenthoate, Phosalone, Pirimiphos ethyl | 0.3-1 mg/kg | (Blasco, Fernández et al.[23] |
| LC-MS/MS | OPP’s & carbamates Amidosulfuron atrazine carbofuran, chlorotoluron Diethofencarb, diethofencarb Dimethoate Fipronil Imidacloprid Isoxaflutole Linuron Methiocarb Methiocarb sulfoxide Metosulam Pirimicarb Rimsulfuron Simazine Hydroxyterbuthylazine, Boscalid, tau-fluvalinate, thiacloprid | 0.2-1232 ng/kg | [17,28,25,14] |
| LC-IT-MS/MS | Fenoxycarb Phenthoate Fonofos Diazinon Phosalone, Pyrazophos Chlorpyrifos methyl, Profenofos Pirimiphos ethyl, Temephos Bromophos ethyl Chlorpyrifos ethyl | 0.024-1.155 mg/kg | Blasco, Vazquez-Roig et al. [21] |
| LC-MS/MS (SRM) | Neonicotinoids (dinotefuran, nitenpyram, thiamethoxam, clothianidin, imidacloprid, acetamiprid and thiacloprid) in honey liqueur | 0.5-1.5 ug/L | (Jovanov, Guzsvány et al. [14] |
| UHPLC-MS/MS | 79 pesticides () | 0.03-1.51 ug/ kg | (Orso, Floriano et al. [34] |
| TLC and video denistometry | Atrazine , simazine | 80, 90 ng/spot respectively | I. Rezic et al [27] |
| ELISA | Imidacloprid and thiamethoxam | Huixin Ma et al [39] |
Table 3: Analytical techniques for analysis of residue of pesticides
Gas chromatography: Gas chromatography has been used with different detectors like electron capture detector (ECD) [24]. ECD is particularly a popular detector due to sensitivity and specificity for electronegative chlorine atoms. It is highly sensitive for halogenated pesticides and nitro compounds. Zacharis et al [9] detected 15 organochlorine pesticides using GC-ECD with LOD (0.02-0.15 ug/L) and linearity (0.986-0.996). It has lower linearity range and widely varying response. GC with microECD (μ-ECD) has revolutionized the trace level detection of halogenated pesticides. It is highly sensitive and reliable detector with low quantification limits. It has broad linearity range for confident quantification, which are lacked by ECD [40,33]. GC-NPD (nitrogen phosphorus detector) is specific for nitrogen-phosphorus containing compounds [24,30].
Flame ionization detector (FID) is a non-specific detector, Amitraz a member of formamidine pesticide family was analyzed by using DLLMEGC-FID approach. The method is linear in range of 0.01-1 mg/kg with limit of detection was 0.0015 mg/kg proving it an efficient method [15]. Mass spectrometric detector (MSD) [20] is termed as the universal detector on the basis of its non-specific properties. MSD being versatile and selective detector is preferred by analyst [5].
Mass spectrometry is frequently used technique for detection, identification and quantification of pesticides due its sensitivity, high selectivity, and low limits of detection, employing atmospheric potential ionization in positive and negative mode [5]. Each mass spectrometer is made up of three main components: (i) an ion source (ii) an analyzer for the separation of ions according to mass-to-charge ratio (iii) a detector to count ions.
Among ion sources electron spray ionization (ESI) and atmospheric pressure chemical ionization (APCI) [23] are mostly used, these both are based on atmospheric pressure ionization. For the determination of multiresidue in honey following analyzers are in common use; (i) ion trap mass analyzer (IT) [9] (ii) time of flight (ToF) [29] (iii) quadropole [41]. Single quadropole analyzer has less separation efficiency not exceeding 3000 now replaced by triple quadropole analyzer. TOF has characteristics of broad range of measurement, high sensitivity and high scanning speed. Its Separation efficiency exceeds 40,000.while the separation efficiency and m/z range of the IT is similar to that of a typical quadropole [41].
There are different MS techniques on the basis of arrangement/ combination of analyzers. Following are the frequently used modifications of the system Tandeem mass spectrometry (MS/MS); it’s a combination of two same or different type of analyzers, and characterized with high separation efficiency, sensitivity and selectivity as compared to single analyzer. It has certain types on the basis of kind of analyzers (a) triple quadropole system (QQQ) (b) quadropole-time-of-flight (Q-TOF) (c) quadroplole-linear-ion-trap (Q-Trap). Amongst three, triple quadropole tandem mass spectrometry is most popular due its higher separation efficiency, higher selectivity and sensitivity [41].
Though gas chromatography is widely used technique with variable detectors but it is only suitable for volatile and less polar compounds or for the compounds which are amenable to derivatization to ensure the volatile properties. Compounds with low thermal stability or low volatility cannot be analyzed by GC. e.g. fluvalinate can be determined through GC-ECD/FID but it is decomposed easily due to higher temperature in GC injector or column, for such compounds liquid chromatography is a preferable technique [5].
Liquid chromatography (LC): To deal with the pesticides which are labile, have not been derivatized, more polar and their metabolites are even more polar and less volatile than the parent compound, for such class of pesticides, liquid chromatography (LC) is used [41-43]. For the separation of analytes HPLC and UHPLC with ultraviolet(UV) (5), Diode array detector(DAD) (12), Variable wavelength detector(VWD) and MS (34) detectors are in common practice. However UHPLC is preferred over conventional HPLC to achieve high eluent flow rate in column and has much greater separation efficiency to determine multicomponent mixtures [41].
LC-MS and LC-MS/MS is an ideal, extremely specific and highly sensitive technique used for identification and quantification of pesticides residues. It provides information about analyte without derivatizing, it can compensate sample purity and it enables simultaneous analysis of the compounds with varying polarity. The only drawback of LC is that it has greater matrix effect thus increasing signal to noise ratio. This problem can be rectified by matrix-matched calibration, internal standard addition and extending the duration of analysis [41].
LC-MS and LC-MS/MS is an ideal ,extremely specific and highly sensitive technique used to detect a wide range of chemicals and a preferred technique over GC-MS because LC-MS involves simple sample preparation and can detect much wide range of pesticides on the other hand GC-MS is limited only for non-polar and volatile class of compounds.
ELISA (Enzyme-linked immunosorbent assay): is a technique in which multiresidues are determined just by simple dilution of the samples, no extraction and clean steps are required. Its results are comparable with LC-MS. Huixin Ma et al., [39] determined the residue of imidacloprid and thiamethoxam in honey through ELISA. In an indirect ELISA microplate wells were coated overnight at 4°C with coating antigens (4 ng of thiamethoxam-BSA or 6ng of imidacloprid-BSA in 100 ml per well of 0.05M carbonate/bicarbonate buffer, pH 9.6) and found recoveries 96- 122%, proved it as an effective method for pesticide residue analysis in honey [39].
Thin layer chromatography (TLC): TLC has been used to determine pesticide residues. It involves extraction of sample with a solvent mixture and separation of the components into blocks with a suitable coating material (e.g. Silica gel) and finally elution with suitable solvents. Rezic et al. [27] detected residues of herbicides atrazine and simazine in honey by this technique with estimated recoveries 92.3% and 94.2% respectively. It is less specific and sensitive technique and requires special equipment for visualization and quantification of results [27].
Miniaturized extraction techniques are preferred over the conventional procedures due to time efficient; reduce solvent consumption and minimal matrix effect. Among the analytical techniques, GC-µECD is best technique for routine analysis of pesticides from honey, while MS detector with either GC or LC is suitable for identification of accaricides and neonicotinoid pesticides from honey. This review helped to judge the suitable technique for determination of volatile and labile pesticides from honey.
Thanks to my colleagues for all the guidance and support.
Download Provisional PDF Here
Article Type: Review Article
Citation: Nazir S, Rafique N, Ahad K (2017) Comparative Evaluation of Extraction Procedures and Chromatographic Techniques for Analysis of Multiresidue Pesticides in Honey. J Environ Toxicol Stu 1(1): doi http://dx.doi.org/10.16966/2576-6430.102
Copyright: © 2017 Nazir S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access