Introduction
More than one million out of a total of 17 million people in the Netherlands suffer from type 2 diabetes mellitus [1,2]. The number of people with the disease is still accumulating and is a growing burden on both societal and individual level, because of the complications like cardiovascular disease and early death [3]. Main risk factors for type 2 diabetes mellitus are overweight and obesity: present in 80 percent of patients with DMT2 [3]. Weight loss is the main strategy to manage type 2 diabetes and this can be achieved by dietary treatment, preferably in combination with physical training [4-7]. Diet therapy and health promotion are effective strategies to lose weight, improve HbA1c, reduce CVD risk factors and diabetes medication and to improve quality of life [4-8]. Patients with type 2 diabetes are also insulin resistant. Weight loss is the best way to reduce insulin resistance [9,10]. The impact of carbohydrates on poor weight loss and poor insulin production is confirmed by the impact that low carbohydrate diets have on weight loss and HbA1c. There is evidence that insulin resistance is treated best with a very low carbohydrate diet [9,10].
The rationale behind administering very low carbohydrate diets is that patients with a large accumulation of abdominal fat are almost all Insulin Resistant (IR), causing high insulin levels, even between meals [8,11]. High insulin levels promote the storage of carbohydrates as triglycerides (lipogenesis) causing weight gain. This is one of the main reasons why patients have trouble to lose weight on diets with normal quantities of carbohydrates, and to maintain their achieved weight loss. Very low carbohydrate ketogenic diets, like the 6 × 6 diet®, reduce the release of endogenic insulin from the pancreas to a minimum, thus causing there lease of triglycerides from the fatty tissue (lipolysis); enhance gluconeogenesis by the liver; and furthermore promote the release of growth hormone, thus raising energy expenditure. The ketones that are formed probably have interaction with ghrelin and leptin, causing lowered appetite [12]. These mechanisms together lead to extensive weight loss [9], and sometimes remission of type 2 diabetes. Protein requirement is >1 gram per kilo present body weight per day, which means it is a high protein diet. High protein intake minimalizes the lost muscle mass; in combination with increased fat intake it produces fast and long-lasting satiety [9,13], reducing craving for food and feelings of hunger, which might contribute to better compliance.
During recent years low carbohydrate diets have become more popular, because of their effect on weight loss, improvement of HbA1c and on the reduction or end of medication [9,10,14,15]. Low carbohydrate diets can vary from a ≤ 10 energy % (20-50 grams) Very Low Carbohydrate Ketogenic Diet to a ≤ 26 energy % (70-120 grams) Low Carbohydrate Diet (LCD) per day [16]. A restriction of less than 50 grams per day is effective on the short term [16,11]. Until now studies have compared the effects of low carb and moderate carb diets on weight loss and HbA1c [13], but the difference between Very Low Carbohydrate Ketogenic Diet and LCD has not been fully established.
Based on the impact of carbohydrates on poor weight loss and increasing HbA1c levels, we hypothesize that diets are most effective on weight loss and HbA1c levels in case carbohydrate intake is lowest. There is one restriction. Studies have revealed that humans need 36 grams of carbohydrate as a minimum level of intake in order to avoid a shortage of glucose in the brain. The carbohydrate need of the brain and the erythrocytes is estimated on 36 grams per day average [11], but there are considerable interpersonal differences [9]. The 6 × 6 dieet® is a VLCKD with 36 grams of carbohydrates per day administered in a strict scheme, meaning that the patient eats 6 times per day 6 grams of carbohydrates, preventing insulin release by the pancreas [10,17]. Preliminary research in Dutch patients receiving the 6 × 6 dieet® showed more weight loss, lower HBA1C and reduction or stopping of medication than the more conventional low carbohydrate diets aiming at higher carbohydrate intake. Inspired by these preliminary results, we decided to compare the 6 × 6 dieet®’s impact on weight loss and HbA1c with the impact of less strict low carbohydrate diet and an energy-restricted diet, which has been common practice during the last decades, among patients with DMT2. Our research question was: “What is the difference in effectiveness between the 6 × 6 dieet® and other low carbohydrate diets and energy-restricted diets for adults (18+) with type 2 diabetes who are overweight or obese regarding to weight loss, improving HbA1c levels, use of metformin, SU-derivatives and insulin at a 3, 6 and 12 month follow-up?”
Methods
Study design
We collected existing data from patients who have been administered the 6 × 6 dieet® and from patients who had been administered a Low Carbohydrate Diet (LCD) or an Energy Restricted Diet (ERD)and carried out a retrospective research to study whether the 6 × 6 dieet® is more effective on weight loss and HbA1c than the LCD and ERD. Some 300 practices of dietitians in the Netherlands use low carb diets for diabetes management [18].
Participants
Two dietitians (WB & AL) from the same center (Dieetzorg Friesland) developed and registered the 6 × 6 dieet®. They have administered the diet to patients from December 2013 onwards until September 2016. Their full data were used on patients being diagnosed with type 2 diabetes mellitus receiving the 6 × 6 diet was used to study the effectiveness of the 6 × 6 diet.
Dietitians administering a Low Carbohydrate Diet (LCD) and/or an Energy Restricted Diet (ERD) to patients with type 2 diabetes across The Netherlands were invited to deliver their data for the purpose of the present study. Members from the Knowledge Centre for Dietitians for Overweight and Obesity (KDOO) were recruited through the KDOO newsletter (https://www.kdoo.nl) and we published a call in the digital newsletter on the website of the Dutch Association of Dietitians (https://www.nvdietist.nl/NieuwsvoorDietisten). The Dutch Association of Dietitians awarded ‘points for accreditation’ for ‘participating in research activities.’ Dietitians that applied, were sent the study protocol by email. Two students from the department of Nutrition and Dietetics of Hanzehoge school, an University of Applied Sciences in Groningen, The Netherlands, carried out the data collection by going to the dietary practices from across the country. Dietitians that took part in the study collected at least 15 subsequent patients per dietitian from September 2016 till 1st of March 2017, who were treated for type 2 diabetes, and who fulfilled the inclusion criteria. AO and BS decided whether an included patient was labeled as being administered an LCD or ERD, based on the carbohydrate intake of the patient as agreed after the first consultation: less than 50 grams in the very low carbohydrate ketogenic study arm, less than 100 grams carbohydrates (≤ 26 en%) in the low carb study arm; more than 100 grams of carbs in the energy restricted study arm, which is in practice also mono and disaccharide and SAFA restricted. Energy restricted diets had more than 100 grams of carbohydrates per day. Ninety percent of dietitians in primary care work with the same software (Evry). Dietitians were asked to collect data in this program. Data were collected on gender, age, body weight and height, HbA1c, use of medication, number of consultations and duration of the treatment in minutes at baseline, 3, 6 and 12 months. Data were also recorded regarding the number of consultations and treatment duration. AO and BS collected these data in situ and uploaded them in Excel.
In and exclusion criteria
Inclusion criteria were: Adults aged 18 years, or older with BMI ≥ 25 kg/m2 and diagnosed with type 2 diabetes, by a general practitioner, defined as fasting glucose ≥ 7.1 mmol/l (127.8 mg/dl) twice or more at several days and/or HbA1c ≥ 43 mmol/l, not necessarily confirmed by lab values for fasting glucose; intake between 01-09-2016 and 01- 03-2017. Exclusion criteria were prednisolone or other corticosteroids, any treatment for oncology, other hormonal treatments, pregnancy, unplanned weight loss by another disease, because of their potential impact on weight change.
Description of diets: Table 1 shows the differences between the three diets. The 6 × 6 dieet® (in this study called 6 × 6) was originally developed by the Dutch Knowledge Centre of Dietitians specialized in Overweight and Obesity in 2013 [10]. The diet comprises of three phases: a first Very Low Carbohydrate Ketogenic Diet phase with 6 × 6 grams of carbohydrates per day; a second phase with a rise in carbohydrates up to 6 × 12 grams of carbohydrates per day (maximum 75 grams); and a maintenance phase with a higher carbohydrate content up to the level that weight loss stops. Dieetzorg Friesland further developed already existing materials for patients and became very strict in the protein content of the diet: at least 1 gram per kilo present body weight. There is no restriction on calorie intake and fat intake and no rules about SAFA’s in the first phase. The diet is administered following a strict protocol and much time is invested in teaching patients how to deal with it in daily life [13]. The low carbohydrate diet (LCD) offers a carbohydrate intake of 50-100 grams per day (equaling ≤ 26 energy %); a protein intake of 1 gram/kg present body weight and 30-35 energy percent fat. The energy-restricted diet (ERD) implies a moderate carbohydrate intake, with restriction of mono- and disaccharides, a protein intake of 0.8 gram/kg ideal body weight and a reduced fat intake of 30-35 energy percent.
| Diet |
Calories |
Protein |
Carbohydrate |
Fat |
Fibre |
|
6×6 dieet® (6×6), a VLCKD |
No calorie restriction |
≥ 1 gram per kg present body weight |
Phase 1: 6 × per day six grams; Phase 2: max 75 grams; Phase 3: individually assessed |
No fat restriction. 1st and 2nd phase: no rules about kind of fat (SAFA; MUFA or PUFA) |
No strict amount. Based on intake |
| Low carbohydrate (LCD) |
No calorie restriction |
1 gram per kg body weight, up to 100 grams per day |
50-100 grams per day |
30-35% of energy% emphasis on MUFA and PUFA |
No strict amount. Based on intake |
|
Energy- restricted (ER) |
Restricted - 600 kcal of usual intake |
0.8 grams per kg ideal body weight or BMI 27 kg-m2 |
≥ 100 grams per day |
30-35% of energy% emphasis on MUFA and PUFA |
30 g/day |
Table 1: Criteria for the 6 × 6 dieet®, low carbohydrate diet and energy-restricted diets.
HbA1c and medication: Data on HbA1c were available for 283 (82.3%) patients at baseline and for 186 (81.9%) patients at time-point 12 months. Patients with missing data on HbA1c were included in all our analyses, except for the analyses on Hb1Ac. Dosage of diabetes medication was recorded regarding metformin, SU-derivates and insulin used 3, 6 and 12 months.
Ethics
We informed dietitians that taking part in the study was voluntary and that their decision to participate or to not participate would not affect their relations with the knowledge center for Dietitians for Overweight and Obesity or the Dutch Association of Dietitians. They were also informed that data would be analyzed and presented anonymously, meaning that dietitians involved in the study and the two students analyzing the data would not be able to present or reveal results on the level of the patient nor on the level of dietitian center. The research leader (TLSV) re-coded all centers and did and will not share the key to these codes. The research leader, not being a dietitian, does not have any relation to the dietitian centers, and he does not know names behind the participant numbers. Asking ethical approval from the medical-ethical committee was not needed, as data had been recorded in the past as part of normal intervention procedures, patients personal data remained unknown to the researchers, and data were analyzed and presented anonymously. Dietitians were free whether to inform their patients that their data had been used. We advised that informing patients was not necessary, as to avoid unnecessary worry amongst patients, and because we assumed that centers lack 100% information regarding patients current address or whether they are still alive. Treatment of all patients had already been finished, and therefore results were not influenced.
Statistical analysis
Baseline characteristics are compared between study arms. Average weight loss and decline in HbA1c between the baseline and time-points at 3, 6, and 12 months were compared between study arms. Amongst those with HbA1c ≥ 43 mmol/mol, we calculated the percentage of subjects who had lowered their HbA1c to <43 mmol/mol and we compared these percentages across study arms. Further, we calculated the percentage of patients stopping or reducing the dosage of their medication and the percentage of patients losing >5% and losing >10% after 1 year, respective to their weight at baseline. We compared these percentages across the study arms. And, we calculated whether the relative weight loss (weight loss as a percentage of initial body weight) differed across study arms, within categories of BMI (BMI 25- 29.9, 30-34.9, and ≥ 35 kg/m2. Comparisons across study arms were tested by Fisher’s Exact test (for binary variables), Pearson Chi-square (for categorical variables), and independent samples T-test, assuming equal variances (for continuous variables). The 6 × 6 study arm was used as the reference study arm in all comparisons.
Intention to treat analysis was performed by calculating the number of patients losing 5% or more from their initial body weight over a year follow-up divided by the number of all 344 patients at baseline starting the diet, including those lost to follow-up, plus those who were initially excluded from the analyses due to stopping diet within a month (n=1, on 6 × 6 diet), hormone treatment from the start (n=1, on 6 × 6), receiving hormone treatment to prevent transplant rejection (n=1, on 6 × 6), starting prednisolone within first three months (n=15, of which 2 were on 6 × 6 diet, 4 were on LCD and 9 were on ERD) or starting prednisolone between months 6 and 12 (n=2, both on 6 × 6 diet). Further, total treatment time and the number of consultations averages between 6 × 6; LC and ER diets were compared. Data were analyzed in SPSS version 25. Statistically significance was considered in case p<0,05.
Results
Data were collected from 16 practices with a wide national dispersal. Figure 1 shows the study flow chart with included patients and drop-out rates. Initially, we collected data of 380 patients. A total of 36 patients were excluded from the analyses. Finally 110 patients on the 6 × 6 diet; 123 patients the LCD; and111 patients the ERD were included in the analysis. Reasons for exclusion were age <18 years (n=1), unknown diet (n=2), insufficient data on body weight or height (n=4), BMI <30 kg/m2 (n=9), stopping the diet within a month (n=1), start of hormone treatment from the start (n=1), medication to prevent transplant rejection (n=1), and starting prednisolone use during the study period (n=17). After 12 months 227 patients (66%) were still in treatment. Drop-out rates were lower in the 6 × 6 (31%) and LCD (30%) study arms than in the ERD (42%) study arm.
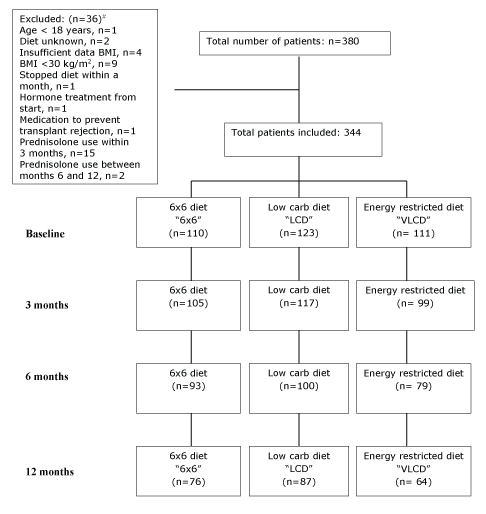
Figure 1: Flow chart of number of patients in the three study arms.
# Notes: The one subject that stopped within a month was on 6 × 6 diet. From the subjects who started prednisolone use between the start and month three, 2 were on the 6 × 6 diet, 4 were on a low carb diet, and 9 were on energy restricted diet. The 2 subjects that started prednisolone use between months 6 and 12 were on the 6 × 6 diet. The 1 subject that started hormone treatment from the start and the 1 subject that started medication to prevent transplant rejection were on the 6 × 6 diet.
The study population is presented in table 2. The percentage of females and age were similar across the three study arms. Patients receiving the 6 × 6 diet had a longer history of type 2 diabetes: 70.0% had type 2 diabetes for 3 years or longer in the 6 × 6 study arm, compared with 56.4% in the LCD and 44.9% in the ERD study arm. Less patients had overweight both in the 6 × 6 and LCD study arms. Mean weight in the 6 × 6 arm was higher (p=0.05). The percentage of BMI higher than 35 kg/m2 and mean BMI were highest in the 6 × 6 study arm, although statistical significance was only reached when comparing mean BMI between 6 × 6 and LCD study arms (p=0.02). More patients in the 6 × 6 study arm were using metformin or insulin. SU-derivates were prescribed equally across study arms. Mean HbA1c at baseline was lowest in the 6 × 6 study arm, statistically significantly compared to mean HbA1c in the LCD study arm: 57.6 and 63.6 (p=0.01); but not in ECD 59.7 (p=0.32).
| |
Data available, n |
6 × 6 |
LCD |
ERD |
Total |
P |
P |
| N=110 |
N=123 |
N=111 |
N=344 |
6 × 6 vsLCD |
6 × 6 vsERD |
| |
|
Percentages |
|
|
| Male/female |
343 |
39/61 |
42/58 |
51/49 |
44/56 |
0.69 |
0.11 |
| Metformin |
333 |
64.5 |
58.8 |
51.9 |
58.6 |
0.42 |
0.07 |
| SU-derivates |
344 |
27.3 |
28.5 |
25.2 |
27 |
0.88 |
0.76 |
| Insulin |
344 |
31.8 |
16.3 |
15.3 |
20.9 |
<0.01 |
<0.01 |
| Years with DM2 |
336 |
|
|
|
|
0.03 |
<0.01 |
| <1 |
15.5 |
29.9 |
30.3 |
25.3 |
| >1t/m <3 |
14.5 |
13.7 |
24.8 |
17.6 |
| >3 t/m <10 |
48.2 |
45.3 |
27.5 |
40.5 |
| ≥ 01 |
21.8 |
11.1 |
17.4 |
16.7 |
| BMI (kg/m2) |
344 |
|
|
|
|
0.48 |
0.09 |
| 25.0-29.9 |
21.8 |
22.8 |
35.1 |
26.5 |
| 30.0-34.9 |
40 |
46.3 |
33.3 |
40.1 |
| ≥ 0.53 |
38.2 |
30.9 |
31.5 |
33.4 |
| |
|
Mean (standard deviation) |
|
|
| Age (years) |
344 |
60.8 (10.9) |
62.1 (10.8) |
62.1 (11.1) |
61.7 (10.9) |
0.38 |
0.41 |
| BMI (kg/m2) |
344 |
34.5 (5.6) |
32.9 (4.5) |
33.1 (5.6) |
33.5 (5.3) |
0.02 |
0.06 |
| Weight (kg) |
344 |
102.2 (17.9) |
97.5 (18.0) |
98.6 (18.6) |
99.4 (18.0) |
0.05 |
0.15 |
| HbA1c (mmol/mol) |
283 |
57.6 (13.5) |
63.6 (18.6) |
59.7 (15.1) |
60.4 (16.1) |
0.01 |
0.32 |
Table 2: Baseline characteristics of the study population.
Weight loss
Patients in the 6 × 6 study arm lost 2.3 (95% CI: 1.1-3.5), 3.0 (95% CI: 1.2-4.7) and 2.7 (95% CI: 0.5-4.8) kg more, after 3, 6, and 12 months, respectively, than patients in the LCD study. Compared to those in the ERD study arm, patients in the 6 × 6 study arm lost 3.9 (95% CI: 2.8-5.1), 3.7 (95% CI: 2.0-5.4) and 3.5 (95% CI: 1.0-6.0) kg, more after 3, 6, and 12 months, respectively.These results are presented in figure 2.
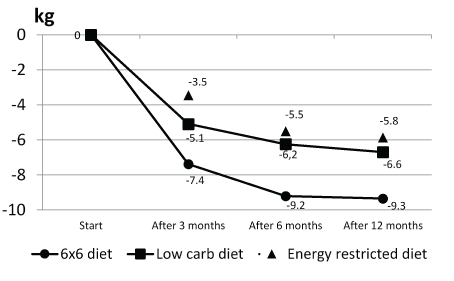
Figure 2: Average weight loss in kilos per diet after 3, 6 and 12 months.
The difference in weight loss between the 6 × 6 diet and the low carbohydrate diet and between the 6 × 6 diet and the energy restricted diet was statistically significant at 3, 6 and 12 months.
The mean relative weight loss, calculated as weight loss divided by body weight at baseline, was 8.7% in the 6 × 6 study arm, which was higher than in the LCD study arm (6,4%, p=0.02) and higher than in the ERD study arm (5.8%, p=0.01). Also, within each category of BMI relative weight loss was higher in the 6 × 6 study arm than in the LCD and ERD study arms, but statistical significance was not reached when comparing these relative weight losses across study arms within BMI categories (Table 3).
| Body mass index (kg/m2) |
| |
25-29.9 |
30-34.9 |
≥ 35 |
All |
| Diet |
| n |
51 |
90 |
86 |
227 |
| 6 × 6 diet |
5.8 (4.5) |
8.1 (7.1) |
10.6 (7.0) |
8.7 (6.8) |
| Low Carbohydrate (LCD) |
4.4 (5.1) |
5.6 (5.3) |
8.4 (5.4) |
6.4 (5.5) |
| Energy Restricted Diet |
5.3 (6.9) |
4.7 (5.5) |
7.4 (6.9) |
5.8 (6.4) |
| p value 6 × 6 vs LCD** |
0.43 |
0.09 |
0.17 |
0.02 |
| P value 6 × 6 vs ERD** |
0.8 |
0.07 |
0.1 |
0.01 |
Table 3: Mean relative weight loss* (standard deviation) after 12 months, within categories of body mass index by diet.
*Relative weight loss is calculated as weight loss divided by body weight at baseline
*100 (%) **p-value calculated by independent samples t-test
Table 4 shows that the percentage of patients losing 5% or more and the percentage of patients losing 10% of their initial body weight were higher in the 6 × 6 study arm than in the LCD and ERD study arms, although differences were statistically significant only when 6 × 6 was compared with ERD, not when compared with LCD. The intention to treat analysis made clear that at least 43.2% of patients in the 6 × 6 study arm lost 5% or more from their weight at baseline; compared with 41.7% (p=0.90) in the LCD and 23.3% (p<0.01) in the ERD study arm. At least 22.9% of patients in the 6 × 6 study arm lost 10% or more from their weight at baseline, compared with 17.3% (p=0.34) in the LCD and 10.0% (p=0.01) in the ERD study arms.
| |
|
Percentage of
patients |
|
Percentage of patients |
| losing weight* |
losing weight |
| |
|
|
|
|
By intention to treat** |
| |
n |
≥ %5 |
≥ %01 |
N |
≥ %5 |
≥ %01 |
| 6 × 6 |
76 |
67.1 |
35.5 |
118 |
43.2 |
22.9 |
| Low Carbohydrate (LCD) |
87 |
60.9 |
25.3 |
127 |
41.7 |
17.3 |
| Energy-restricted (ERD) |
64 |
43.8 |
18.2 |
120 |
23.3 |
10 |
| P by Fisher’s Exact 6 × 6 vs LCD |
|
0.42 |
0.17 |
|
0.9 |
0.34 |
| P by Fisher’s Exact 6 × 6 vsERD |
|
0.01 |
0.04 |
|
<0.01 |
0.01 |
Table 4: Percentage of patients losing weight 5% or more and losing 10%
or more from their initial body weight across the study arms.
* For those with full data on body weight at baseline and at 12 months.
**Calculated as the number of patients losing ≥ 5 or 10% weight divided by the number of all 344 patients at baseline starting the diet, including those lost to follow-up, plus those who were initially excluded from the analyses due to stopping diet within a month (n=1, on 6 × 6 diet), hormone treatment from the start (n=1, on 6 × 6), receiving hormone treatment to prevent transplant rejection (n=1, on 6 × 6), starting prednisolone with in first three months (n=15, of which 2 were on 6 × 6 diet, 4 were on LCD and 9 were on ERD) or starting prednisolone between months 6 and 12 (n=2, both on 6 × 6 diet).
HbA1c
Figure 3 shows that the improvement of HbA1c was similar in the 6 × 6 and in the LCD study arm at 3, 6, and 12 months. At 12 months, the decline in LCD study arm was 2.6 (95% CI: -2.5-7.7) mmol/mol larger than in the 6 × 6 study arm, although the average level was lower in 6 × 6 (63.6/49.7 LCD, and 57.6/47.1 6 × 6 respectively). The decline of HbA1c in the 6 × 6 study arm was statistically significantly larger than in the ERD larger at 3 and 6 months, not at 12 months. At time-point 12 months, decline in HbA1c in the 6 × 6 study arm was 1.6 (95% CI: -3.9-7.0) mmol/mol larger than in the ERD study arm.
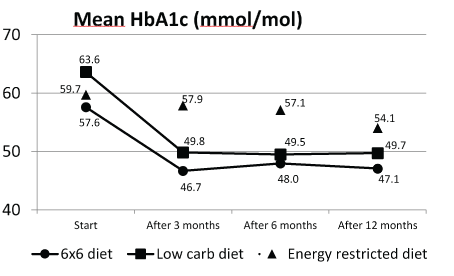
Figure 3: Decline in HbA1c in the 6 × 6; LC and ER diets.
Amongst patients with HbA1c of ≥ 43 mmol/mol at baseline, 34.5%, 20.7%, and 9.3%, had lowered their HbA1c to < 43 mmol/mol at timepoint 12 months in the 6 × 6, LCD, and ERD study arm, respectively (6 × 6 versus LCD p=0.14; 6 × 6 versus ERD p<0.01) (data not shown).
Medication
Table 4 shows the reduction in medication use per study arm. The reduction in the use of Metformin was highest in the 6 × 6 study arm at 3, 6, and 12 months, although not reaching statistical significance when comparing with the LCD study arm at 3 months. The reduction in use of SU-derivates was also highest in the 6 × 6 study arm, but only reaching statistical significance when comparing with the ERD study arm, at 3, 6, and 12 months (Table 5). After 12 months, reduction in insulin use was highest in the 6 × 6 study arm, but only reaching statistical significance when comparing with ERD.
| |
6 × 6 |
Low Carbohydrate |
Energy Restricted |
P* |
P* |
| Diet |
Diet |
6 × 6 vsLCD |
6 × 6 vs ERD |
| Metformin |
| 3 months |
24.6 |
16.2 |
8 |
0.29 |
0.03 |
| 6 months |
45.3 |
25.9 |
13.3 |
0.04 |
<0.01 |
| 12 months |
62 |
28 |
25.6 |
<0.01 |
<0.01 |
| SU derivatives |
| 3 months |
90 |
66.7 |
37.5 |
0.06 |
<0.01 |
| 6 months |
86.7 |
66.7 |
41.7 |
0.13 |
<0.01 |
| 12 months |
90 |
73.3 |
41.7 |
0.18 |
<0.01 |
| Insulin |
| 3 months |
85.7 |
90 |
88.2 |
1 |
1 |
| 6 months |
85.7 |
85 |
70.6 |
1 |
0.26 |
| 12 months |
88.6 |
75 |
58.8 |
0.26 |
0.03 |
Table 5: Percentage of patients that stopped or reduced use of medication
at 3, 6, and 12 months.
* P by Fisher’s Exact test
Number of consultations and duration of treatment: Data on number of consultations and treatment duration were available for 333 patients. Patients on 6 × 6 visited their dietitian 7.5 times during the follow-up period. Patients on LCD and ERD visited their dietitian 5.7 times (p<0.01) and 5.4 times (p<0.01), respectively.
Average total treatment duration was 305 minutes (5.08 hours) for patients on 6 × 6, which was 89 (95% CI: 66-113) minutes longer than on LCD, and 89 (95% CI: 62-115) minutes longer on ERD.
Discussion
This retrospective study makes clear that weight loss after 12 months was greater in patients on a 6 × 6 dieet® than in patients on a Low Carbohydrate Diet (LCD) or an Energy Restricted Diet (ERD). Decrease in HbA1C levels was larger in the 6 × 6 study arm than in the ERD study arm. More patients in the 6 × 6 study arm had stopped or reduced their intake of metformin SU derivates, and insulin after 12 months than in the LCD and ERD study arms.
Dispersal of the study population
Because the Netherlands is a small country, where the low carbohydrate diet has already spread out to most dietary and medical practices, our study population can be seen as representative for primary healthcare in the whole country.
One drawback of delivering data by invitation is that dietitians may be more willing to participate in case they feel comfortable regarding their own success. If this phenomenon is true, we argue that our selection procedure has led to an under-estimation of the differences with the 6 × 6 diet rather than to an over-estimation. Hence, all data available regarding the 6 × 6 diet have been used, whereas data on the other two diets were retrieved by invitation.
One drawback of our selection procedure regarding study-arms is heterogeneity between the arms, despite targeting a specific patient population: diabetic patients with overweight. As body weight and body mass index values differed across study-arms, we have also presented results per BMI-category. It is important to note that these BMI-specific analyses did not lead to other insights regarding the effectiveness of the diets.
Impact on weight loss
The positive results on weight loss are in line with a systematic review that found a 2.5 kg difference in weight loss between very low carbohydrate and moderate carbohydrate diets in patients with DMT2 after 3-6 months [17]. In our study patients on 6 × 6 had 2.7 kg more weight loss compared to LCD and 3.5 kg greater weight loss compared to ERD after 12 months. The difference in weight loss in our study in favor of 6 × 6 is still present at that time-point, in contrast to most trials where these differences have diminished after a year [17, 19, 20]. Looking at the mean relative weight loss related to diet and BMI, it is clear that 6 × 6 shows the best results in patients with a BMI ≥ 30/kg/ m2 . In patients with BMI 24.9-29.9/kg/m2 differences in weight loss are smaller. The intention to treat analysis reveals a ≥ 5% weight loss in 43.2% of patients on 6 × 6, and a ≥ 10% weight loss in 22.9%, which clearly is more than on LCD (41.7 and 17.3%), and is significantly more than on ERD (23.3 and 10.0%).
To explain why 6 × 6 has the best weight loss results a combination of factors can be held accountable. First of all the strong restriction of carbohydrates on 6 × 6, allowing patients 6 times per day a meal with 6 grams of carbohydrates, during the first phase which can last six months, lowers glucose levels in the blood and insulin secretion by the pancreas, thus changing energy metabolism causing triglycerides to be used as main energy source, leading to weight loss. Weight loss can improve insulin sensitivity by changing the function of adipose tissue [21]. Secondly, a very low carbohydrate diet allows patients to eat more fat and more protein, which have more satiety than carbohydrates, partly because they slow down stomach release due to longer digestion time; and partly because more ketones are produced, which also reduce appetite [12,22,23]. These effects have been reported by patients as pleasant: patients have the feeling they can control their appetite, which is often new to them. Thirdly, high protein intake also leads to better preservation of muscle mass, which stimulates energy expenditure, also leading to more weight loss.
Because weight loss, and more specifically the loss of visceral and abdominal fat, is so important in type 2 diabetes management, the differences in outcomes on these three diets should not be put aside lightly. Recently it was suggested to raise the criteria for weight loss in patients with type 2 diabetes to 10 kg for prevention of comorbidities [24]. Moreover, there is a strong relationship between weight loss and glycaemia [24]. A weight loss of 10 kg may mean the difference between having a chronic disease and being in remission; or less use of medication, and postponing or stopping insulin treatment. Patients should therefore be encouraged to set their weight loss goal as high as possible.
Reduction of HbA1c
Lower levels in HbA1c are a sign of improving type 2 diabetes. The larger reduction of HbA1c induced by the 6 × 6 dieet® and by the LCD than by the ERD is in line with Avenell A, et al. review concluding that in patients with type 2 diabetes a diet with less than 50 grams of carbohydrate per day was more effective to lower HbA1c than a low carb diet of more than 50 grams or an energy-restricted diet after three months [25]. These outcomes were supported by Elhayany A, et al. who found that weight loss and HbA1c were significantly reduced on a low carb Mediterranean diet [26] and Sainsbury E, et al. systematic review that low carbohydrate diets were more beneficial for lowering HbA1c than moderately restricted or high carbohydrate diets [17]. Reduction of HbA1c is a well known phenomenon in low carb diets for DMT2 with ≤ 26 en%, which equals <100 grams per day, compared to high carbohydrate diets [27], and confirms our findings that there is a direct relationship between the carbohydrate content of the diet and the HbA1c. On the contrary, a systematic review comparing low and high carbohydrate diets indicated that a low fat high carbohydrate intake significantly increased fasting insulin, and lowered HDL cholesterol compared with a high fat low carb diet, thus enhancing the risk of insulin resistance [28].
Reduction of metformin, sulfonylurea derivatives (SU) and insulin
Our findings show a significant reduction in DMT2 medication after 12 months which is larger in the 6 × 6 study arm than in the LCD and ERD study arms, although only statistically significant compared to ERD. The decision to reduce or quit medication is dependent on reduced HbA1c levels. We even found remission of DMT2 is possible, as defined by HbA1C <43 mmol/mol: 34.5% of patients in the 6 × 6 study arm reduced their HbA1C to <43 mmol/ mol. In The Netherlands SU-derivatives are the second step in DMT2 management, when metformin is not effective enough [29], leading to an extra weight gain of 1-3 kg, because of the promotion of insulin release from the pancreas. In fact, SUs work contrary to the aim of weight loss [30]. A low carb diet should always be accompanied by a total stop of SUs, to promote weight loss. Insulin is administered when SUs do not work effectively enough, leading to more weight gain as well [30]. The benefit of the 6 × 6 dieet® is that the use of insulin, even in patients with more than 10 years of DMT2, could be reduced or even stopped, because the very few carbohydrates in the diet are all used for the brain and erythrocytes.
Professional guidance and adherence to the diet
Very low carbohydrate ketogenic diets can lead to deficiencies in micronutrients and fiber, when not carried out well. Patients therefore need the appropriate guidance of a dietitian [31-34]. Patients in the 6 × 6 had extra consultations to help them get used to the diet; especially getting used to the high protein, strict low carbohydrate regimen took time, leading to longer treatment time. Loss to follow-up was larger in the LC and ER study arms than in the 6 × 6 study arm. Adherence is likely to be influenced positively by successful weight loss, especially in patients that have a long weight loss history with little success. The good results in glucose values and the reduction of medication are also positive points reported by patients. Reasons for drop out can be disappointing weight loss results, lack of motivation, personal problems, or taking a sabbatical [20]. Patients have numerous weight loss cycles and relapse often, before they reach a point where they can maintain a stable, healthy weight [35]. In a survey of adherence to diet, patients with type 2 diabetes and their health professional each viewed barriers differently [36] patients expressed a dislike for foods included in meal plans, contrary to the HPs who considered social environment as a more important barrier. We think therefore, that a longer treatment time and a flexible attitude of the health professional/ dietitian is needed to keep the patient in care and motivated. In 6 × 6 patients had to go by a strict structure, but were free in their choice of foods, under the condition that they were low in carbohydrates. They did not feel the diet as a restriction. Meat, cheese, butter, and several high protein snacks, e.g. fish and sausage were allowed, giving patients a lot of freedom in their social life. This may have led in combination with rather rapid weight loss to better adherence. The fact that medication could be reduced, creating a feeling that someone’s health is improving, is also a possible motivator.
Measuring insulin
Insulin resistance is the main reason for prescribing low carbohydrate diets, but is not diagnosed in primary care practice by measuring fasting insulin, but indirectly by measuring other values, such as: BMI; waist circumference; weight loss history; reporting carbohydrate cravings; and the presence of hypertension; dyslipidemia; or impaired glucose tolerance. To target the right patients for VLCKD and LCD diets, we need to measure fasting insulin. A study by Ter Horst KW, et al. showed that 78% of obese men had higher fasting insulin, 110 versus 63 pmol/l (15.8 mIU/L versus 9.0 mIU/L) and that >74 pmol/l (10.6 mIU/L) was a cut-off point for insulin resistance with good sensitivity and specificity in research and in clinical situations [37]. Based on fasting insulin values, we can set specific recommendations for carbohydrate content in diets for patients with IR; DMT2; and other comorbidities.
Recommendation for practitioners
A diet that reduces the release of insulin, meaning a low carbohydrate ketogenic diet, leads to weight loss and restoration of insulin sensitivity in patients with type 2 diabetes. We therefore advocate to treat patients with type 2 diabetes first with a low carbohydrate weight loss diet, preferably as low as less than 50 grams per day, to see to what extend the normal glucose metabolism in the body can be restored; and only when this approach does not lead to improved glucose values start with medication.
Strengths and weaknesses
Strengths of this study are the length of the study, the national dispersal and the strict protocol of data collection. The fact that our study was not a carefully designed RCT but an observational study from every day dietary practice makes it possible to implement the results in other patient settings, especially in primary care. The repeatability of the study and the principles of the diet in daily practice are high.
Weaknesses were that we did not systematically measure energy intake and energy expenditure per patient. We collected glucose values and HbA1c levels from the physician’s office, and did not measure them ourselves. Some patients had lower HbA1c levels than 43 mmol/ mol at baseline: in 6 × 6 (n=10, 9.9%); in LCD (n=6, 6.0%); and in ERD (n=4, 4.9%) at baseline, which may have influenced positive outcomes on the number of patients reaching the ≤ 43 HbA1C level in the 6 × 6 study arm.
Conclusion
The findings of this retrospective study show that the 6 × 6 dieet®, a very low carbohydrate ketogenic diet with high protein and no fat restriction, when consistently performed, produces larger weight loss, reduction of HbA1c, reduction of diabetes medication in overweight or obese patients with type 2 diabetes than a milder low carbohydrate diet or an energy-restricted diabetes diet.
Acknowledgements
The authors wish to thank all dietitians for their willingness and cooperation in the data collection. They wish to thank M. Sealy at Hanze Hogeschool for her feedback on earlier versions of the manuscript.
Conflict of Interest
The authors declare no conflict of interest. No one has been paid or sponsored to collect data, process them, or write the article.
Authorship
EG designed the initial 6 × 6 diet (2013), made the study design and wrote the article. AO and BS collected all data. AO, BS, and TLSV carried out the analyses. WB and AL developed the 6 × 6 dieet® and wrote the study protocol. All authors read, corrected and approved of the article.
Data Sharing
Data described in the manuscript, codebook, and analytic code will be made available upon request.




