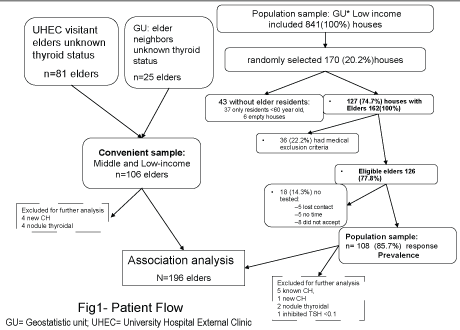
Figure 1: Patient recruitment flow chart


Lilia Cárdenas-Ibarra1* Perla Alejandra Mellado-Urbina1 Ana Laura Turner-Llaguno1 Adolfo Montemayor-Alatorre1 Javier Ramos-Cárdenas1 José Carlos Lira-Castillo1 Raúl Fernando Gutierrez Herrera2 Jesús Zacarías Villarreal-Pérez1
1Endocrinology Service, Dr. José E. González University Hospital and School of Medicine, Autonomous University of Nuevo León, Nuevo León, México*Corresponding authors: Lilia Cárdenas-Ibarra, Endocrinology Service, Dr. José E. González University Hospital and School of Medicine, Autonomous University of Nuevo León, Nuevo León, México, Tel: +52 81 83485764/7871; E-mail: dralilia@gmail.com
Objective: To determine the prevalence of hypothyroidism and concomitant Metabolic Syndrome (MS) in a formerly goiter endemic middle-low-income urban area with a population over 59 years of age.
Methods: Two cross-sectional samples were obtained: 1) Population Sample (PS): 108 elders from randomly selected homes of a middlelow income urban community, and 2) Convenience Sample (CS): 106 middle-low income urban elderly. Thyrotropin (TSH), Thyroid Peroxidase Antibodies (TPOAb) and Free Thyroxin (FT4) were measured by electrochemiluminescence immunoassay. A TSH ≥ 4.0 µIU/L with FT4 in normal range was considered Subclinical Hypothyroidism (SH), while with a low FT4 was considered Clinical Hypothyroidism (CH).
MS components: Adiposity, Hypertension (HTN), Dyslipidemia (DL), and hyperglycemia; treatment deems component as positive. Prevalence with 95% Confidence Intervals (CI) estimated in PS; associations and Logistic Regression (LR) used the PS and the CS.
Results: PS had 35 (32.4%, CI: 23.6-41.2%) subjects with elevated TSH; six (5.5%, CI: 1.2-9.9%) had CH, and 29 (26.9%, CI: 18.5- 35.2%) SH. SH was positively associated with high TPOAb, HTN, DL treatment (kDL), High Total Cholesterol (HtCh), and symptoms (dry skin+constipated+cannot lose weight+puffy eyes), 27.0% vs 3.8%, χ2 =23.1 P<0.001, 88.9% vs 69.9% χ2 =8.4 P<0.01; 66.7% vs 42.9% χ2 =9.7, P<0.001; 76.2 vs 39.8%, χ2 =22.6, P<0.001; 92.1 vs 67.7% χ2 =13.8, P<0.01, respectively. SH was indifferent to gender, oldest old, high Triglycerides (TG), Low High-Density-Lipoproteins (HDL), diabetes; but LR showed SH inversely associated to HDL, and HbA1c, and directly associated to symptoms, HTN and HtCh, all P<0.01, while TG, age and gender remained insignificant. Elders with SH had BMI 31.5 ± 7.0 vs 29.0 ± 5.1 kg/m2 , P<0.01, and a lower FT4: 1.1 ± 0.2 vs 1.3 ± 0.2 ng/dL, P<0.01. By LR: symptoms, HTN, kDL, HtCh, predicted SH correctly, 75.5%, with a sensitivity of 71.4 and a specificity of 77.4.
Conclusions: The prevalence of hyperthyrotropinemia was higher than expected by TPOAb. SH was positively associated with HTN, kDL, HtCh, low HDL and obesity but negatively with hyperglycemia, suggesting hyperglycemia could reduce TSH as prolonged fasting does.
Elderly; Subclinical hypothyroidism; Prevalence; Metabolic syndrome
MS: Metabolic syndrome; PS: Population sample; CS: Convenient sample; TSH: Thyroid stimulating hormone; TPOAb: Thyroid peroxidase antibodies; FT4: Free thyroxine; SH: Subclinical hypothyroidism TSH ≥ 4.0 and normal FT4; CH: Clinical hypothyroidism TSH ≥ 4.0 and low FT4; CI: Confidence interval; df: Degrees of freedom; LR: Logistic regression; Low-HDL: High-density-lipoprotein <40 in men and <50 for woman; HTN: Arterial hypertension; SBP: Systolic blood pressure.
Thyrotrophic hormone levels reflect thyroid function [1]. Hallowell reported that 4.2% of Mexican-Americans 12 years of age or older have thyrotrophic hormone (TSH) levels>4.5 µIU/mL. Also, the 2.5, 50 and 97.5 percentiles of TSH were 0.43, 1.36 and 3.91 µIU/L, respectively, in the MexicanAmerican reference group. In disease-free individuals 70-79 years of age, elevated thyroid peroxidase antibodies (TPOAb) were present in 21.8%. In these increased TSH reaches 12%; if TPOAb positive subjects are excluded it only reaches 6% [2]. Hypothyroidism prevalence varies according to environmental and genetic factors [1]. In the elderly, hypothyroid symptoms may be obscured by concomitant morbidities and/or confused by changes in aging [3,4]. On the other hand, damage to target tissues by subclinical hypothyroidism has also been documented [5,6].
The elderly population in Mexico is estimated to increase four-fold by 2050 [7]. The data on hypothyroidism prevalence is scarce. As elderly form the fastest growing population, this poses more difficulties in identifying and reaching a diagnosis of hypothyroidism; therefore, to promote health and avoid deterioration in function, screening is paramount. Monterrey, a city of Mexico, is a former goiter endemic region [8]. This was overcome by using iodinated salt. The implementation of iodinated salt started in 1942 with the last update in the year 2000, NOM-040-SSA1-1993 [9]. Nevertheless, two studies that evaluated hypothyroid prevalence had conflictive findings. Salinas-Martinez R et al. [10] in 2004, reported clinical hypothyroidism in 2% of retired workers from a local industry, but in a study of a local geriatric outpatient clinic, a TSH level>4.5 µIU/L was found in 29.7% (CI 20.8-38.6%) patients with dementia, and in 27.3% (CI: 12.1-42.5%) of patients without dementia [11]. The former seems to have had a higher cutoff point. Also, since the subjects studied were retired workers and the second group was geriatric patients, the results cannot readily be extrapolated to the general population. On the other hand, Metabolic Syndrome (MS) has been determined to be highly prevalent in this region [12].
While screening is the best option to plan management of elderly health [6], an effort must be made to avoid false positives since severe comorbidities can distort thyroid function test results. MS abnormalities are high in individuals of Mexican descent and also increase with age [13,14]. Diabetes mellitus and MS have been found to be associated with hypothyroidism [15,16]. Thus, the objective of this study was to determine the prevalence of hypothyroidism and metabolic syndrome abnormalities in elderly population from a former endemic goiter area.
A cross-sectional design was used with two samples of elderly. A randomly selected Population Sample (PS) of 108 elderly individuals from houses in a low-income urban community (Geostatistic unit: 1902600011584 [7]), and a convenience sample (CS) of 106, also middle-low income, elders (81 elders visiting patients at the “Dr. Jose Eleuterio González” University Hospital, and 25 community elders wanting to participate but who were not residents in the PS selected houses) (Figure 1).
The target population was urban middle-low income men and women older than 59 years from Monterrey, Mexico. Inclusion criteria were, besides age, income and accepting to be screened, the PS also had to be residents of the randomly selected houses.
Exclusion criteria were the presence of dementia, stroke, cancer, an arrhythmia or a severe heart, lung or kidney condition or a behavioral problem such as severe alcoholism; the CS additionally excluded elders with a previous diagnosis of thyroid disease.
At least 95 individuals were needed to find a prevalence of 14%, a precision of 7% and a confidence of z=1.96 [17]. This is in the range of reported prevalence in elderly Mexican Americans by Hallowell [1].
For the PS, a list of 841 houses was obtained from the aforementioned community. One-hundred seventy houses (20.2%) were randomly selected and visited to enlist and enroll eligible elderly. A community module was set up for physical examination and blood drawing from October to November 2008 and from September to November 2009. Subjects could also go to the University Hospital for measurements.
For the CS, subjects were recruited by printed advetisements in the visiting areas of the University Hospital and medical students approaching elders face-to-face. Elder neighbors of the PS subjects who were willing to be tested were also enrolled. All volunteers were seen at the University Hospital for tests, a short clinical history, and a physical exam. Laboratory measures were performed at the Endocrinology Service of the University Hospital. The recruiting period started in October 2008 and ended in May 2010.
Subclinical hypothyroidism was considered when a serum TSH value ≥ 4.0 µIU/L was found and concurrent free thyroxin levels were within normal range. Clinical hypothyroidism was declared if TSH value ≥ 4.0 µIU/L and free thyroxin level was below normal range or when the individual was taking levothyroxine [2]. For MS components, ATP III, WHO and AACE criteria were considered [18], as follows: adiposity use BMI ≥ 25 k/m2 as WHO and AACE include it, also BMI correlates with waist circumference, r꞊0.825, in our population [19]; hyperglycemia assessed by HbA1c ≥ 6%: previously diagnosed DM or new HbA1c>7%, and Glucose Intolerance (GI) would be HbA1c between 6 and 7% inclusive; blood pressure ≥ 130/85 mmHg for hyperglycemic elders and ≥ 140/90 mmHg in normoglycemic; low High-density-lipoprotein (HDL)<40 in men and<50 in women, high Triglyceride (TG) ≥ 150 mg/dL; treatment deems component as positive.
TSH and FT4 [20] were measured by Electrochemiluminescence Immunoassay (ECLIA) with a Cobas E601 analyzer (Roche Diagnostics GmbH, Mannheim, Germany). The TSH working range is 0.005-100.00 µIU/mL; the expected normal values for the 2.5th and 97.5th percentile are 0.270-4.20 µIU/L; and for FT4 0.93-0.7 ng/dL. TPOAb [20] were measured by ECLIA with an Elecsys 2010 analyzer (Roche Diagnostics). The measuring range is 5.0-600 UI/L; the 95% of normal was 34 IU/L.
MS: HbA1c was measured by the immunoturbidimetric test (One-HbA1c FS) via Star-Dust MC15 both from DiaSys. Its Coefficient of Variation (CV) is 1.6%; blood glucose level was calculated by the American Diabetes Association equation: (28.7* HbA1c) -46.7꞊AG mg/dl. Lipids: triglycerides, and High-Density-Lipoprotein (HDL) were determined manually with RANDOX reactive GPO-PAP and precipitation with phosphotungestic acid/MgCl2 respectively; total cholesterol (t Chol) was determined by CHOL reagent with the SYNCHRON system (Beckman Coulter); total CV was 4.5%; Low-DensityLipoproteins (LDL) was calculated from measured values of total cholesterol, triglycerides and HDL according to: LDL mg/ dL꞊total chol–HDL-(TG/5) for TG under 400 mg/dl. Blood pressure measurements were made with the patient seated comfortably, with the back supported, legs uncrossed and with no talking or smoking; two measurements were made allowing a rest time with a HoMedics sphygmomanometer with a largeadult arm cuff. Body Mass Index (BMI) was body weight in kg with height in m2 using the Ultimate 2204 scale (TANITA) and a fixed vertical metric ruler with the subject barefoot and in light clothes.
Data on age, gender, morbidity (for PS-included thyroid diagnosis) and current medication were collected; 10 closedended questions on thyroid symptoms and a physical exam were also applied [1]. This study was also used to identify elderly subjects with mild subclinical hypothyroidism for a trial to determine if thyroxin results in health improvement. All phases and informed consent were approved by the Ethics Committee of the Hospital (EN08-028) and the study is registered in clinicaltrials.gov (NCT00921050).
Analysis was performed using SPSS version 17.0. The graph for TSH distribution uses only the population sample. The prevalence by sample is given with a 95% Confidence Interval (CI). To evaluate associations, data from PS and CS were pooled by Chi square for gender, TPOAb, hypothyroidism symptoms, and components of MS. Also, Mantel-Haenszel odds ratio (MH-OR) and Logistic Regression (LR) were used to assess predictability of SH by MS and symptoms; for continuous variables Student’s t-test and the Mann-Whitney U test were used. The levels to consider significance were P<0.05, and a trend for P<0.1 [17].
PS. In the 170 sampled houses, we identified 162 elderly inhabitants; 53 (33.8%) were men. Thirty-six (21.7%) had exclusion criteria. Among the excluded subjects, there were 19 men (Χ2 ꞊8.5, df=1, P<0.01). Of the 126 eligible subjects, 108 had thyroid function status determined (103 underwent lab tests and five more were on levothyroxine); thus, the study response was 85.7%. There were 27 men and 81 women for analysis. Mean age was 68.51 ± 7.54 years with a mean BMI of 29.9 ± 5.7 kg/m2 and a systolic/diastolic blood pressure of 143.9 ± 29.5/86.6 ± 15.4 mmHg.
TSH interval distribution is shown in figure 2. The left end bar corresponds to a subject with an inhibited TSH, followed by 4 subjects with TSH borderline subnormal. To the right there are 30 elders with TSH levels equal or greater than 4.0 µIU/L. The bimodal shape of figure 2 agrees with 4.0 µIU/L as a cutoff point for the curves. The five subjects, with a known diagnosis already on levothyroxine, were not included in the figure. Thus, there were 35 subjects (32.4%, CI95% 23.6-41.2%) with thyroid sub function. Also, figure 2 depicts TSH distribution by three age groups (60-69, 70-79 and 80+y/o); there were 20 (30%), 12 (40%) and 3 (25%), respectively in the increased levels with no significant difference by age.

Figure 1: Patient recruitment flow chart
In the CS, there were 106 elders. Their TSH distribution by age and age groups was similar to the PS with 64 having TSH in normal range (0.4 to 3.9 µIU/L), 34 with SH (4.0 to 9.9 µIU/ mL), 4 new CH (lowFT4 or TSH ≥ 10 µIU/L), and 4 (3.8%) elders with a palpable but small thyroid nodule. These last eight elders were excluded to merge CS and PS for SH association analysis.
In the pooled sample, there were 196 elders: 56 (28.6%) were male, 70 (35.7%) were older than 69 years, 63 (32.1%) had SH, 22 (11.2%) had raised TPOAb, 149 (76%) had HTN (112 known plus 37 new HTN), 99 (50.5%) kDL, 100/147 (68.0%) had high total cholesterol (HtCh>200 mg/dl), 86/147 (58.5%) had high-TG, 111/147 (75.5%) low-HDL, 133/147 (90.5%) had high-TG and/or low-HDL, 124/147 (84.4%) had kDL and/ or HtCh (kDLHtCh), 139/147 (94.6%) had kDL and/or lowHDL; adiposity (BMI ≥ 25.0 kg/m2 ) was found in 141 (71.9%), DM in 73 (37.2%: on treatment 66 cases and 7 new diagnosis [HbA1c>7%]) and another 45 (23.0%) elders had glucose intolerance (GI, HbA1c between 6 and 7% inclusive).
A comparison of euthyroid and SH elders is shown in table 1. There were no significant differences regarding age, gender, blood glucose, TG and HDL but SH had significantly higher tCh, LDL levels and BMI. The mean Systolic Blood Pressure (SBP) was significantly higher in SH, while, Diastolic Blood Pressure (DBP) mean difference did not reach significance but a trend is seen as SH had more often DBP>85 mmHg (P꞊0.08). An increase in TPOAb was associated with SH; in addition, FT4 and TT4 levels, while in normal range, were significantly lower in SH than in euthyroid elders.
The distribution of metabolic components by diagnosis is also displayed in table 1. HTN, and kDL were directly associated to SH, while kDM seem indifferent but showed a negative trend when stratifying for HTN and kDL, then MHOR꞊0.54 (CI: 0.27 to 1.09) P<0.1. Also, HbA1c (cutoff 6.5%) instead of diagnosis, there were more SH with an HbA1c below 6.5% than over it, reaching significance with a MH-OR꞊0.34 (CI: 0.16 to 0.72) P<0.01. Counting missing HbA1c as <6.5% provides a more stringent but still significant MH-OR꞊0.43 (CI: 0.21 to 0.89) P<0.05. Neither HDL mean nor percent with low HDL were significantly different in euthyroid vs SH elders but logistic regression showed a negative relation; that is, SH had lower HDL values: -0.09 ± 0.04, P<0.01. The other predictors in the equation were HTN 1.7 ± 0.54, P<0.001, HtCh 1.6 ± 0.53, P<0.001, any symptom (dry skin+constipated+cannot lose weight+puffy eyes) 1.72 ± 0.56, P<0.001 and hyperglycemia (HbA1c>6.5%)-1.14 ± 0.43 all P<0.05. Other candidate variables not reaching significance were age, BMI, triglycerides; last step-2 Log likelihood 149.1, Nagelkerke R2 ꞊0.353, omnibus tests of model coefficients Χ2 =43.2, df꞊5, P<0.001.
| TSH | Euthyroid 0.4 to 3.9 µIU/L |
SH 4.0 to 9.9 µIU/L |
Test | MWZ |
| N (%) | 133 (100) | 63 (100) | ||
| Age, years | 67.9 ± 0.7 | 68.7 ± 1.0 | t꞊-0.7 | 0.7 |
| Gender male, n (%) | 42 (31.6) | 14 (22.2) | Χ2꞊1.8 | 1.4 |
| High TPOAb, n (%) | 5 (3.8) | 17 (27.0) | X2꞊-23.1‡ | 4.6‡ |
| FT4, ng/dL | 1.3 ± 0.04 | 1.1 ± 0.02 | t꞊4.6‡ | 4.4‡ |
| TT4, nmol/L | 107.1 ± 3.7 | 95.5 ± 2.1 | t꞊2.7‡ | 2.7‡ |
| Glucose, mg/dl | 165 ± 6.6 | 150 ± 6.9 | t꞊1.6 | -2.97♦‡ |
| tCh mg/dl | 213 ± 4.9 | 230 ± 5.8 | t꞊-2.2† | 2.2† |
| LDL, mg/dl | 136 ± 4.6 | 158 ± 6.3 | t=-2.8‡ | 2.5† |
| Triglycerides, mg/dl | 178 ± 9.1 | 187 ± 13.1 | t꞊-0.6 | -0.3 |
| HDL mg/dl | 42.5 ± 1.0 | 41.0 ± 0.7 | t꞊1.08 | -0.09▼‡ |
| Systolic, mmHg | 142 ± 2.1 | 157 ± 4.2 | t꞊3.1‡ | 2.7‡ |
| Diastolic, mmHg | 87 ± 1.4 | 91 ± 2.2 | t꞊1.4 | 1.3 |
| BMI, kg/m2 | 29.0 ± 0.5 | 31.5 ± 0.9 | t꞊-2.5† | 2.0† |
| HTN, n (%) | 93 (69.9) | 56 (88.9) | X2꞊8.4‡ | -2.9‡ |
| kDL, n (%) | 57 (42.9) | 42 (66.7) | X2꞊9.7‡ | -3.1‡ |
| kDM, n (%) | 21 (33.3) | 52 (39.1) | Χ2꞊0.61 | 0.53§* |
| Low HDL n (%) | 66 (74.2) | 45 (77.6) | Χ2꞊0.22 | n.s. |
| TG>150mg/dL n (%) | 52 (58.4) | 34 (58.6) | Χ2꞊0.01 | n.s. |
Table 1: Comparison of normal TSH range and SH
Values are mean ± standard error or n (%). For FT4 and TT4: n꞊38 normal vs 63 SH; for lipid levels n꞊89 vs 58 SH; kDL: on antilipidemic; kDM: Known Diabetes Mellitus type2; Tests: Student’s t test; MWZ: Z value of Mann Whitney U test.
LR. ♦There were less HS with HTN+kDL+kDM
▼by LR: HDL+tCh+HTN+Sx+HbA1c predicted SH. n.s.꞊no significant by LR
§꞊MH-OR꞊0.53 (CI: 0.26 to 1.096).
Glucose꞊28.7 *HbA1c-46.7
Significance: *P<0.1, †P<0.05, ‡P<0.01
Table 2 depicts symptom frequency distribution among a normal range of TSH and SH. Global frequencies were 25.3% to 57.3% but only four of the assessed symptoms showed a statistically significant predominance in SH elders (dry skin+constipated+cannot lose weight+puffy eyes), maintaining significance after adjustment for HTN and kDL. The presence of one or more of the mentioned significant symptoms was positive in 148 (76.7%) elders showing a significant predominance in SH.
| TSH 0.4 to 3.9 µIU/L |
SH 4.0 to 9.9 µIU/L |
Frequency 196 (100) | Χ2 | M-H Χ2 | ||
| N (%) | 133 (100) | 63 (100) | ||||
| 1 | Sweatless | 35 (26.7) | 14 (22.2) | 49 (25.3) | 0.46 | 0.3 |
| 2 | Coarse voice | 42 (32.1) | 18 (28.6) | 60 (30.9) | 0.24 | 0.1 |
| 3 | Paresthesia, cramps | 71 (54.2) | 40 (63.5) | 111 (57.2) | 1.5 | 1.0 |
| 4 | Dry skin | 58 (44.3) | 36 (57.1) | 94 (48.5) | 2.8* | 2.7* |
| 5 | Constipation | 47 (36.2) | 33 (52.4) | 80 (41.5) | 4.6† | 4.7† |
| 6 | Cannot lose weight | 35 (26.7) | 31 (49.2) | 66 (34.0) | 9.6‡ | 6.5‡ |
| 7 | Moves slowly | 37 (28.2) | 23 (36.5) | 60 (30.9) | 1.4 | 2.3 |
| 8 | Coarse skin | 39 (29.8) | 22 (34.9) | 61 (31.4) | 0.5 | 0.4 |
| 9 | Puffy eyes | 32 (24.4) | 31 (49.1) | 63 (32.5) | 11.9‡ | 7.1‡ |
| 10 | Cold hands | 28 (21.4) | 20 (31.7) | 48 (24.7) | 2.5 | 0.1 |
| Any: 4, 5, 6, 9 positive | 90 (69.2) | 58 (92.1) | 148 (76.7) | 12.4‡ | 12.5‡ | |
Table 2: Symptoms distribution by TSH 4.0 cutoff
TSH: Thyroid-Stimulating Hormone; SH: Subclinical Hypothyroidism; M-H: Mantel Heanszel χ2
controlling for HTN and kDL
Any of following Sx꞊dry skin+constipated+cannot lose weight+puffy eyes.
Significance* p<0.1, † p<0.05, ‡ p<0.01
Table 3 shows observed and expected SH by the presence of one or more of the above-mentioned symptoms controlling for kDL plus HTN: 0꞊neither HTN nor kDL, 1꞊either HTN or kDL, and 2꞊both HTN and kDL); Thus symptoms were significantly associated with SH tested by MH-OR of 6.6 (CI: 2.3 to 18.6). In agreement, binary logistic regression identified: HTN, kDL and the presence of any of these symptoms were identified as predictors of SH by forward stepwise selection; The significant variables at the last step were: HTN, kDL, and any of the above symptom; the equation was correct 75.5%, the specificity 77.4% and sensitivity 71.4%; then adding HbA1c (>6.5꞊1 and <6.5꞊0), increased the times being correct to 80.1%, and specificity 94.0% but decreased sensitivity to 50.8. The last step model had: -2 Log likelihood꞊189.9, Nagelkerke R2 ꞊0.35, and omnibus test model coefficients: Χ2 =56.3, df=4, P<0.001; Beta ± SE were any symptom (dry skin+constipated+cannot lose weight+puffy eyes)꞊1.96 ± 0.54, P<0.001, HTN 1.48 ± 0.5, P<0.001, kDL=2.2 ± 0.46, P<0.001, high HbA1c꞊-0.94 ± 0.39, P<0.02, and Constant-4.77 ± 0.8, P<0.01. Variables not reaching significance were age and BMI.
| N at Risk | Observed SH | Expected | |||||||
| HTN+kDL | Any Sx | No Sx | Total | Any Sx | No Sx | Total | Any Sx | No Sx | Total |
| Neither | 15 | 8 | 23 | 1 | 0 | 1 | 0.7 | 0.3 | 1 |
| Either | 58 | 15 | 73 | 12 | 1 | 13 | 10.3 | 2.7 | 13 |
| Both | 75 | 25 | 100 | 45 | 4 | 49 | 36.7 | 12.3 | 49 |
| Total | 196 | 58 | 5 | 63 | 47.7 | 15.3 | 63 | ||
Table 3: SH by hypertension (HTN)+dyslipidemia (kDL) and any symptom (Sx) (dry skin+constipated+cannot lose weight+puffy eyes) N꞊196
Any Sx: dry skin+constipated+cannot lose weight+puffy eyes.
MH Χ2 ꞊14.2, df꞊1, P<0.001, OR꞊6.6, (CI: 2.3 to 18.6)
In this population, the bimodal distribution of TSH agrees with 4.0 µIU/L as a cutoff point to distinguish two populations (euthyroid and hypothyroid). The prevalence found for CH as well as for SH was higher than that reported in Mexican American elderly population. The excess of hypothyroidism was unjustified by TPOAb, cutoff point, or prescription drugs. HS was not associated with gender, medication or COPD, but had an inverse association with hyperglycemia, and a direct association with kDL, HtCh, low HDL and HTN. Iodine insufficiency cannot be discarded. HTN plus kDL plus at least one of the following symptoms: dry skin+constipated+cannot lose weight+puffy eyes, correctly predicted 75.5% of SH or normal TSH range.
The hypothyroidism prevalence found exceeds that reported in Mexican American elders [2]; with a 4.5 µIU/L cutoff point in the PS there were 25 (23.1%) SH and 6 (5.6%) CH, together there were 31 (28.7%, CI: 20, -37.2%) elders with hypothyroidism. The lower CI limit is above the upper limit for Mexican American elders 60-69, 70-79 and 80+years old, all under 16% [2].
On the other hand, raised TPOAb was associated with a TSH ≥ 4.5 µIU/L in both PS and CS, with a pooled OR꞊11.8, CI: 4.7-29.0, in agreement with other reports [2,21,22]. An elevated TPOAb was also higher in women without reaching significance; however, the overall TPOAb frequency was 27 (12.7%, CI: 8.2 to 17.1%); i.e., two thirds of elders with hypofunction were TPOAb negative. The proportion of elevated TPOAb was 14.6, 10.7 and 9.5% for 60-69, 70-79 and 80 and older, respectively, Χ2 =0.8, p>0.1. Nevertheless, in Mexican Americans both TPOAb and TSH increased with age. The proportion of elevated TPOAb was greater than hypothyroidism prevalence at each age group [2]. Unlike them, the SH prevalence in our study surpassed the percentage of high TPOAb. Also, SH prevalence showed no increase, while TPOAb decreased, without reaching significance.
Another difference can be seen in total thyroxine in our disease-free elders with TSH between 0.4-4.49 µIU/L; for comparison, the 2.5, 50.0 and 97.5 percentiles of thyroxine in men were 59.2, 94.0 and 168.9 nmol/L, and in women 64.0, 103.0 and 150.3 nmol/L, while the respective percentiles in Mexican Americans [2] for men were 70.4, 111.8 and 159.3 nmol/L and for women 71.4, 119.8 and 178.3 nmol/L; for all ages, thyroxine does not decline with age [23]. It is clear that all Mexican American values are significantly above the respective values for men and women in the present study. A comparison of a Mexican American reference population versus our sample using 4.0 µIU/L as the cutoff point provides the same conclusion, that in the disease-free normal, the TSH thyroxine level is lower in this study than in Mexican Americans. The elders with SH in our study had significantly lower thyroxine levels than the elders with TSH in normal range. The prevalence found and the thyroxine difference between SH and normal TSH are in agreement with a previous report on subclinical hypothyroidism in an outpatient geriatric clinic from Monterrey, Mexico [11]. The prevalence found is also in agreement with the SH prevalence in elderly reported in the Colorado thyroid study [24] and the acknowledged prevalence in elderly Spanish women [25].
The controversies of the cutoff point are still unsettled [26] while in United States population the upper TSH normal limit increases with age [27], in other countries with borderline sufficient iodine intake, it declines [28]. In this study, a serum TSH of 4.0 µIU/L better distinguished the two TSH distribution curves (population with hypothyroidism on the right side of figure 1). As Völzke H et al. [29] concluded, normal TSH ranges in formerly iodine deficient regions are different than those without a scarcity of iodine. Moreover, the TSH value of 4.0 µIU/L is in accord with the 97.5 percentile of the NHANES III Mexican-American reference group of 3.9 µIU/mL [2]. Indeed, the mean total thyroxine and free thyroxine were significantly lower in the group with SH (TSH ≥ 4.0 µIU/L) than the group with TSH (between 0.4 to 3.99 µIU/L inclusive). Thus, evaluations for association use the 4.0 µIU/L cutoff point for SH.
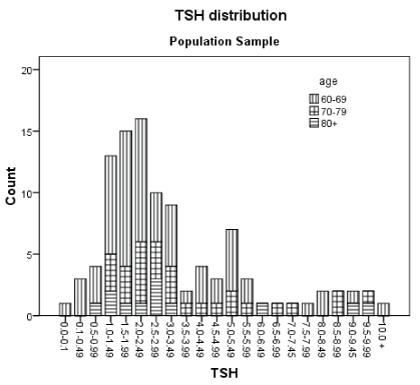
Figure 2: TSH: Thyroid Stimulant Hormone in µIU/L. Five subjects with a previous diagnosis and in treatment with levothyroxine was not included.
The components for metabolic syndrome were highly prevalent: 76% HTN, 63.3% dyslipidemia, 71.9% overweight (BMI ≥ 25 kg/m2) and 59.2% with kDM or glucose intolerance, all similar to previous local reports [12,30] but higher than that reported in the Mexican National Health survey 2012 (ENSANUT [13]), probably because the survey identifies only previously diagnosed cases and does not include data on hypothyroidism in adults. Another study in central Mexico [31] reported a higher prevalence of MS components than ENSANUT, but still lowers than our prevalence of MS components, which could be due to the age of the subjects studied; their sample had younger subjects. The mean age in Garduño-Garcia Jde J et al. [31] was 42.3 ± 10 years versus 68.5 ± 7.5 years in our study.
In agreement with the literature indicating increased vascular resistance and arterial stiffness in hypothyroidism [32,21,24], our data showed an association of SH with HTN. The mean SH systolic blood pressure (SBP) was significantly higher than in elders with TSH in normal range. The mean DBP was also higher in SH not reaching significance, yet a DBP>85 mmHg was found more often in SH than in euthyroid elders (54.0 vs 42.1%, P<0.1 by one-tailed Fisher’s exact test), suggesting vascular rigidity as is described in clinical hypothyroidism [21]. Garduno-Garcia Jde J et al. [31] also reported increased SBP in SH and an insignificant elevation in DBP.
Elders with SH had a significantly more frequent diagnosis of dyslipidemia and/or increased total cholesterol and LDL levels, a finding in agreement with Garduno-Garcia J et al. [31]. This is also in agreement with the reported association of dyslipidemia with hypothyroidism in the literature [21,24].
An elevated BMI was often found in elders with SH without reaching significance for neither the 25 nor the 30 kg/m2 cutoff points. Nonetheless by t-test and the Mann-Whitney U test elders with SH were statistically significantly heavier (~2.4 kg/m2) than elders with TSH in normal range. This difference agrees with the modest weight lost seen with levothyroxine treatment in clinical hypothyroidism [33]. In addition, other sources of adiposity, such as hyperglycemia, could obscure this association in our study sample size.
On the other hand, hyperglycemia showed an inverse association with SH revealed through MH-OR꞊0.43 (CI: 0.21 to 0.89) P<0.050. As well, Garduño-Garcia Jde J et al. [31] reported no glycemic difference between SH and euthyroid subjects. This diverges from a Greek report on DMT2 patients having a higher prevalence of thyroidal abnormalities, including hypothyroidism, than nondiabetic subjects [34]. But merging different thyroidal illnesses confuses associations; for example, the Greek report that thyroid dysfunction associated to LDL with an OR of 0.99, CI 0.987 to 0.997 [35]; that is, lower levels of LDL for thyroidal dysfunction than euthyroid diabetics, while in this and other studies, hypothyroidism was associated with higher levels of tCh, LDL, and low levels of HDL [21,24].
In our study, SH was found in 31.9% of elders with DMT2 and 32.4% in nondiabetic elders. Ravishankar SN et al. [35] also reported an SH prevalence of 31.2% in elders with DMT2, but did not study nondiabetics. A Scottish study of patients with diabetes mellitus (both types) reported an association with thyroid disorders of 13.4%, higher in DMT1 and lower in men with DMT2 6.2%; all higher than nondiabetics [36]. Moreover, in the present study, in urban elder citizens, data showed a rather inverse association between SH and hyperglycemia. It brings to mind that prolonged fasting reduces TSH with no change or a small increase in Ft4 [37] as seen in our hyperglycemic studied subjects. In DM, while blood glucose is high, cells are still starving as in prolonged fasting.
Consequently, our data point to an association of SH with metabolic syndrome components, but in relation to vascular origin and adipose tissue rather than insulin resistance as suggested by the inverse association to hyperglycemia, increased body weight, and the direct association with SBP, lower levels of HDL, and high tCh and LDL. On the other hand, TG was not associated with SH probably because TG is associated with hyperglycemia, which is related to cell starving that decreases TSH levels, thus obscuring mild thyroid hypofunction. Grundy SM et al. [18] also pointed out that advancing age probably affects all levels of pathogenesis of the metabolic syndrome.
Certainly, age could change the scenario, hypothyroid symptoms are more difficult to discern in elders than in younger mature adults [3,21] In this study, symptom frequency ranged from 25 to 57% but only four (dry skin+constipated+cannot lose weight+puffy eyes) of the ten assessed, were significantly found in SH. In the Colorado study, most symptoms were associated to SH but they included a large proportion of younger mature adults. Anyway, those symptoms had low sensitivity to predict SH as in our study. This agrees with the asymptomatic notion due to unspecific and confusion with the aging process and/ or other health problems [7,8]. However, by binary logistic regression, the presence of any of the four significant above mentioned symptoms and controlling for HTN+kDL improved prediction, mainly sensitivity maintaining reasonable good specificity and predictive values.
No subject in our sample was on lithium or amiodarone or a beta blocker, there were 18 elders with mild COPD distributed indifferently to SH. The excess of prevalence cannot be attributed to those pharmacological agents or COPD. Spurious measurement was discarded as all thyroid tests were performed in duplicate and 55% of the subjects (all elders with TSH out of normal range, or elevated TPO plus 38 elders in TSH normal range, age and gender matched to SH group) had a second thyroid profile 2 to 4 months after the first test with a correlation coefficient 0.965, P<0.001. The source of prevalence excess is not clear. Regional variations could be the reason for these discrepancies [29,38]. Endemic goiter was prevalent in the studied region half a century ago and it is currently abated only with iodinated salt [8], which has been widely available in urban and rural areas of Nuevo Leon for more than 50 years [9]. Besides prolonged iodine deficit intake, thyroid hypofunction may come from an excess of iodine intake by a direct inhibitory effect or by eliciting autoimmunity [21-23,38]. The studied city has very hot weather year around. In such a hot environment people tend to increase salt intake and thus iodine intake. Then later in life, the recommended salt restriction to control HTN makes them prone to iodine deficiency since there is no other iodine fortified food as available as salt in our locality. HTN was found in 76% of our study population with most (80%) being already a long time in salt restriction [30]. The observed lower total and free thyroxine levels, the moderate proportion of elders with elevated TPOAb, and the high hypothyroidism prevalence suggest, to some extent, iodine deficiency [39], albeit urinary iodine was not measured nor morphologic thyroidal studies were done.
The study group lacks power to find a significant gender or old vs oldest old difference. Even if the seven eligible men and the eleven women not participating were included as normal, the confidence intervals still overlap 15.6% (CI 3.0-28.2%) for men vs 28.2% (CI 18.7-37.8%) in women. It would seem that aging tends to decrease the gender gap of SH prevalence. There was no decline in the SH prevalence in the oldest old. The relative proportion in this age group in the sample was as expected for low-income Mexican elderly [7]; thus, oversampling to increase power would also increase survival bias. Cause-effect is beyond our cross-sectional design.
Hypothyroidism prevalence in urban elders from Monterrey Mexico is greater than in Mexican-American elderly (33.3% vs 14%) with lower frequency of high TPOAb. Metabolic syndrome is associated with SH directly by HTN, lower HDL and high tCh and indirectly with hyperglycemia, suggesting a greater involvement of vascular disorders than insulin resistance in this population. Concomitant high blood pressure, kDL and the presence of at least one of the associated symptoms (dry skin+constipated+cannot lose weight+puffy eyes) can be used to focus screening when economic resources are scarce. In low iodine regions, an alternative source of dietary iodine must be assured to provide adequate iodine intake in individuals with hypertension. Surveillance of their urinary iodine level, thyroid status, and blood pressure control should also be carried out. Further study is needed to increase our understanding of the SH associations with metabolic syndrome components and to determine adequacy of iodine consumption.
We thank Sergio Lozano-Rodriguez, MD for his help in reviewing the manuscript. We also thank the medical students, Martin Mendez Guerra, Aram Aleman Nava, Arturo E Colín Tovar, Andrea Ileana García Pisanty, Gerardo Loa Aguirre, Nelly Marlen Nava Rodriguez, Sofia Athié Moreno Fuentes, Francisco Javier Murguía Nuñez, Roberto Mauro García Torres and Erick Fabian Morales Macías for their help in recruiting subjects.
The authors declare that they have no conflict of interest.
The study was supported with the Endocrinology Service’s own economic resources.
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Cárdenas-Ibarra L, Mellado-Urbina PA, Turner-Llaguno AL, Montemayor-Alatorre A, Ramos-Cárdenas J, et al. (2018) Hyperthyrotropinemia Prevalence and Metabolic Syndrome Estimates in Elders in a Formerly Endemic Goiter Area. Int J Endocrinol Metab Disord 4(1): dx.doi. org/10.16966/2380-548X.145
Copyright: © 2018 Cárdenas-Ibarra L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access