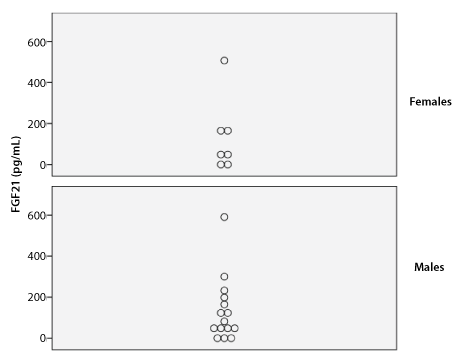
Figure 1: Serum Concentration of FGF21


Filippina Filia Dimitriadi1 Shufang Wu1 Wei Yang1 Bruce Bernstein2 Francesco De Luca1 Rita Ann Kubicky1*
1Section of Endocrinology and Diabetes, St. Christopher’s Hospital for Children, Drexel University College of Medicine, Philadelphia, PA, USA*Corresponding authors: Rita Ann Kubicky, MD, St. Christopher’s Hospital for Children, 160 E. Erie Avenue, Philadelphia, PA 19134, USA, Tel: (215) 427-8100; E-mail: ritaann.kubicky@tenethealth.com
Background: Fibroblast Growth Factor 21 (FGF21) mediates metabolic adaptation to fasting in rodents and humans. As previously demonstrated, increase in FGF21 causes Growth Hormone insensitivity and growth failure. We hypothesized that serum FGF21 in short prepubertal children is positively associated with serum GH levels and negatively with serum Insulin-like Growth Factor-1, height, weight, and growth velocity.
Methods: We prospectively enrolled short prepubertal children in our section. Serum FGF21, growth hormone and Insulin-like Growth Factor-1 were measured. Anthropometric parameters were collected. Body Mass Index and growth velocity were calculated.
Results: 22 patients were recruited (mean age: 5.6 ± 2.9 years). Mean FGF21 level was 132.5 pg/mL. No significant gender difference was identified in median FGF21 levels.
An inverse association was identified between serum FGF21 and growth velocity (p=.043) and Height Z-score (p=.046) in females.
Conclusion: This is the first study reporting circulating FGF21 levels in prepubertal males and females. Our results suggest an inverse correlation between serum FGF21, Height Z-score and growth velocity in females.
Fibroblast growth factor 21; Growth failure
GH: Growth Hormone; IGF-1: Insulin-like Growth Factor-1; FGF21: Fibroblast Growth Factor 21; FGF: Fibroblast Growth Factor; FGFR: Fibroblast Growth Factor Receptor; WAT: White Adipose Tissue; BAT: Brown Adipose Tissue; FR-KO: Food Restricted Knock Out; FR-WT: Food Restricted Wild Type; BMI: Body Mass Index; RIA: Radioimmunoassay.
Malnutrition/undernutrition is the most common cause of growth failure in the world. Interestingly, in addition to the developing countries with the highest prevalence of stunted growth, many American children experience an inadequate nutrient intake in the first 3 years of life, that leads to underweight and short stature [1]. In mammals, statural growth is primarily regulated by Growth Hormone (GH) and Insulin-like Growth Factor-1 (IGF-1), with IGF-1 synthesis being mostly induced by GH itself [2].
In rodents, fasting leads to reduced GH secretion as well as hepatic GH sensitivity [3-5]; in humans, malnutrition also induces liver GH insensitivity but, unlike rodents, is associated with increased circulating GH levels [6-8].
Underweight-short children often exhibit elevated GH and low IGF-1 blood levels, which reflect a state of GH resistance [9]; yet, little is known on the causative mechanisms of these hormonal changes.
FGF 21 (Fibroblast Growth Factor 21) is a member of a subfamily of FGFs (including FGF15/19 and FGF23) which lack the FGF heparinbinding domain [10,11]. Thus, these FGFs can diffuse away from their tissue of synthesis and function as endocrine factors [12,13]. They bind the FGF receptors (FGFR1-4), which are tyrosine kinases. To activate the receptors, FGF21 must interact with β-Klotho, a single-pass transmembrane protein. FGF21 binds to β-Klotho through its C-terminus, while it binds to FGFR through its N-terminus. The co-localization of β-Klotho and FGF21 in white adipose tissue (WAT), brown adipose tissue (BAT) and in the liver is consistent with the notion that FGF21 regulates mammalian metabolism primarily in these three tissues/organs. Recently, we have shown that FGF21 and its signaling system are also expressed in the rodent growth plate. Indeed, in cultured chondrocytes isolated from 3-week-old mouse tibial growth plates and from late fetal mouse metatarsal growth plates, we demonstrated mRNA expression of FGF21, FGFR1, FGFR3, and β-Klotho, and protein expression of FGFR1, FGFR3, and β-Klotho [14].
During reduced caloric intake, mammals experience an increased synthesis of Fibroblast Growth Factor 21 (FGF21) [15]. Increased activity of FGF21 in the liver induces gluconeogenesis, fatty acid oxidation, and ketogenesis: as a result, FGF21 is considered a key regulator of the metabolic adaptation to fasting [15-22].
Increased expression of FGF21 during food restriction in rodents appears to inhibit longitudinal bone growth [23]. We have recently shown that mice with FGF21 gene deletion undergoing food restriction (FR-KO mice) exhibit attenuated reduction of their body linear growth and tibial growth, and similar weight loss, compared to food-restricted wild-type mice (FR-WT) [14]. In addition, FR-KO mice have higher serum IGF- 1 and lower serum GH levels compared to FR-WT mice: these findings indicate that mice with FGF21 gene deletion remain more GH sensitive than wild-type mice during food restriction [14]. These data implicate a causative role for FGF21 in malnutrition-associated growth failure, possibly by inducing growth hormone resistance.
In humans, recently published data indicate that serum FGF21 levels are inversely related to growth rates in infancy, suggesting that FGF-21 is a negative regulator of human statural growth early in life [24,25]. However, little is known on the functional role of FGF21 in the growth regulation of older children, especially in those with underweight and short stature.
We hypothesized that:
In order to test our hypothesis, the objectives of this study were:
Study approval was obtained from the Drexel University Institutional Regulatory Board (Protocol 1212001718). Informed consent and assent (if indicated) were obtained from parents and patients upon enrollment.
Subjects were recruited prospectively from the outpatient clinics of the Sections of Endocrinology and General Pediatrics at St. Christopher’s Hospital for Children between February 2013 and January 2015.
The inclusion criteria were:
Subjects with the diagnosis of chronic disorders, or pubertal stage of ≥ Tanner 2 (≥ Tanner 2 breast development in girls and testicular size ≥ 4 cc in boys) were excluded.
Underweight (based on CDC definition) was defined by Weight for Length of <5th percentile for subjects aged less than 2 years and BMI of <5th percentile for older subjects.
After enrollment, a non-fasting blood sample was collected to measure GH, IGF-1 [GH was measured by a 2 site immunoenzymometric assay (TOSOH) at St. Christopher’s Hospital for Children Central Laboratory, while IGF-1 was measured in a commerical laboratory (Lab Corp) and by RIA after acid:alcohol extraction] and FGF21 (measured using an enzyme linked immunosorbent assay kit by MilliporeSigma; catalogue number: EZHFGF21-19K; Merck KGaA, Darmstadt, Germany) at one of the coauthors’ research laboratory.
Anthropometric measurements, including Weight, Height and BMI, were obtained. Growth velocity was calculated using two height measurements, taken 3-6 months apart. In many subjects, both measurements were taken at the outpatient clinics of the Sections of Endocrinology and General Pediatrics at St. Christopher’s Hospital for Children; in others, the first measurement was taken at the referring pediatrician’s office, while the second one was taken at St. Christopher’s Hospital for Children. Percentiles and Z-scores were calculated based on reference values for the CDC’s 2000 growth charts (downloaded from http://www.cdc.gov/growthcharts), and the Children’s Hospital Boston Growth Calculator 2.01. Detailed medical and family histories were obtained.
Data were analyzed with IBM SPSS software version 20.0. Data (including the anthropometric measures) were expressed as mean ± standard deviation (SD) and median for the serum FGF21 levels. A p value of <0.05 was considered to be statistically significant. Spearman’s rank order correlations (r) were used to assess associations between the different variables and serum FGF21 levels. Mann Whitney U test was used to assess difference in FGF21 levels between genders.
A total of 22 children with growth failure aged 1-10 years were recruited (Table 1). All of the subjects were prepubertal. The majority of the subjects were males and either African-American or other.
| Age, mean ± SD | 5.6 ± 2.9 years |
| Male/ Female gender, n (%) | 14/8 (63/37) |
| Race, n (%) | |
| Hispanic | 2 (9) |
| African-American | 8 (36) |
| Caucasian | 5 (23) |
| Other | 7 (32) |
| Height Z-score, mean ± SD | -2.6 ± 0.85 |
| BMI Z-score, mean ± SD | 0.01± 1.03 |
| Growth Velocity Z-score, mean ± SD | -0.5 ± 1.97 |
| Table 1: Patient Characteristics (N=22) | |
All of the subjects (n=22) met the criteria for Growth Failure (as defined in the Materials and Methods) and 9% (n=2) of the subjects were underweight (as defined in the Materials and Methods).
Median serum GH and IGF-1 levels were 3.68 ng/mL and 89.31 ng/mL, respectively. Median serum FGF21 level was 69.95 pg/mL (range: 0-591 pg/mL) in males and 62.80 pg/mL (range: 0-507 pg/mL) in females. There was no significant difference in FGF21 levels between genders based on the Mann Whitney U test (p=.99) (Table 2 and Figure 1).

Figure 1: Serum Concentration of FGF21
| Males | Females | |
| Mean | 131.06 | 135.56 |
| Median | 69.95 | 62.80 |
| Minimum | 0 | 0 |
| Maximum | 591 | 507 |
Table 2: Serum FGF21 levels (pg/mL)
p value=.99 (Mann Whitney U test)
An inverse, statistically significant association was identified between serum FGF21 and Growth Velocity (p=.043) as well as Height Z-score (p=.046) in females.
Spearman’s rank order tests identified no significant associations between serum FGF21 levels and age, time of the day, other anthropometric parameters including weight (z-score), BMI (z-score).
In addition, no significant correlations were found between serum FGF21 levels and serum IGF-1 or GH levels (Table 3).
| Males | Females | |||
| Spearman coefficient |
p | Spearman coefficient |
p | |
| Age | -.492 | .074 | .564 | .187 |
| Time | -.329 | .251 | -.527 | .224 |
| BMI % | .183 | .589 | .551 | .257 |
| BMI Z-score | .183 | .589 | . 551 | .257 |
| Height | -.413 | .142 | .509 | .243 |
| Height Z-score | .152 | .603 | -.764 | .046 |
| Growth velocity | .183 | .531 | -.771 | .043 |
| Growth velocity Z-score | .101 | .768 | -.725 | .103 |
| Weight | -.369 | .195 | .564 | .187 |
| Weight Z-score | .148 | .614 | .673 | .098 |
| IGF-1 | -.271 | .370 | .127 | .786 |
| GH | .453 | .120 | .291 | .527 |
Table 3: Correlations between serum FGF21 level and other variables Spearman rank order correlation. Correlation is significant at the 0.05 level (2-tailed)
To our knowledge, our study is the first one to report circulating levels of FGF21 in prepubertal, males and females with short stature. We have demonstrated an inverse, statistically significant correlation between serum FGF21 and height z-score (p=.046) as well as growth velocity (p=.043) in prepubertal girls, suggesting that FGF21 might be negatively affecting the regulation of statural growth in humans. No significant difference was identified in serum FGF21 levels between genders (Mann Whitney U test, p=.99), which is in contrast to a previously reported higher serum FGF21 levels in boys [26].
Previous evidence has suggested a regulatory role for FGF21 in mammalian growth. In 2008, Inagaki et al. [27] showed that transgenic mice overexpressing FGF21 are smaller and exhibit shorter tibial bones when compared to that of WT mice. They have also shown that overexpression of FGF21 decreased GH signaling activation and, in turn, IGF-1 expression in the liver, indicating that FGF21 may be implicated in the regulation of growth by causing GH insensitivity.
Food restriction in rodents is associated with reduced skeletal growth due, at least in part, to increased FGF21 activity. Indeed, we have shown that, in mice, chronic undernutrition results in an increased synthesis and activity of FGF21 both systemically and locally within the growth plate. As a result, FGF21 inhibits GH action by acting both as an endocrine and a paracrine factor [14,28].
Published evidence of an effect of FGF21 on statural growth in humans is limited. In adults affected by anorexia nervosa, serum FGF21 was directly correlated with GH and inversely correlated with IGF-1 levels [29]. In peripubertal boys with idiopathic short stature, the authors found no correlation of serum FGF21 with anthropometric measures including height, weight, growth velocity, BMI or serum IGF-1 levels [30].
A regulatory role for FGF21 on longitudinal/statural growth appears more obvious in infancy. Mericq et al. [25] studied a cohort of preterm and term infants. During the first 6 months of life, in the whole group serum FGF21 was inversely correlated to the body linear growth, and such a significant inverse correlation persisted in the 6–12-month period in the group of preterm infants born with a gestational-age-appropriate birth weight. Term infants who experienced catch-up growth in the first 6 months of life exhibited lower serum FGF21 levels at 6 months compared to those who did not experience catch-up growth. Thus, these findings indicate that FGF21 in infancy is inversely correlated with linear growth rate, thus suggesting that FGF21 is a negative regulator of human growth.
In another study, the authors evaluated only premature infants [24]. Consistent with Mericq’s findings [25], a significant negative association between FGF21 level during the first 5 weeks of life and change in length was found. No association was found between serum IGF-1 and change in length. Furthermore, these authors evaluated the effects of FGF21 on GH action in cultured chondrocytes. Consistent with our findings in cultured mouse growth plate chondrocytes [28], the addition of recombinant FGF21 to the culture medium of human chondrocytes reduced the GHmediated induction of chondrocyte proliferation. Therefore, these findings suggest that FGF21 may inhibit longitudinal bone growth by antagonizing GH action in human chondrocytes.
In conclusion, the results of our study support an inhibitory regulatory role for FGF21 in the statural growth of prepubertal girls; however, we were unable to demonstrate an FGF21-mediated induction of GH insensitivity possibly leading to growth failure. The small size of our sample, the limited number of underweight children enrolled, as well as the lack of a control group limit the impact of our findings. Yet, these findings justify the need for additional longitudinal studies on a larger cohort of prepubertal short and normal statured children with a wider BMI range (including underweight children).
Download Provisional PDF Here
Article Type: Research Article
Citation: Dimitriadi FF, Wu S, Yang W, Bernstein B, De Luca F, et al. (2017) Undernutrition and Growth Failure: Is Fibroblast Growth Factor 21 (FGF21) The Missing Link? Int J Endocrinol Metab Disord 3(1): doi http://dx.doi.org/10.16966/2380- 548X.131
Copyright: © 2017 Dimitriadi FF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access