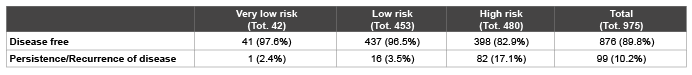
Table 1: Subdivision of patients into three risk categories according to European Thyroid Cancer Taskforce [2].

Federico Arecco1 Francesca Bardesono1 Stefania Corvisieri1 Ilaria Messuti1 Barbara Puligheddu1 Carlotta Sacerdote2 Arnoldo Piccardo3 Marco Tampellini4 Riccardo Pellerito5 Fabio Orlandi1
1Endocrine Unit, Humanitas - Gradenigo Hospital, University of Turin, Turin, Italy*Corresponding author: Prof. Fabio Orlandi, MD, Section of Endocrinology, Humanitas – Gradenigo Hospital, University of Turin, Humanitas - Gradenigo Hospital, Corso Regina Margherita 10, 10153, Turin, Italy, Tel: +39 011 8151395; Fax: +39 011 8151589; E-mail: fabio.orlandi@unito.it
Background: The follow-up of differentiated thyroid cancers is based on neck ultrasonography and serum thyroglobulin assay (Tg), during l-T4 therapy and after recombinant human TSH administration; this test appears quite expensive, considering that only a small percentage of patients with undetectable Tg on TSH suppression therapy shows a response after TSH stimulation.
Objectives: The aim of our study was to verify whether low levels of serum thyroglobulin at the time of remnant ablation (A-Tg) associated with undetectable thyroglobulin levels on TSH suppression (S-Tg), have sufficient negative predictive value for recurrence of disease, thus avoiding rh-TSH test in Differentiated Thyroid Cancer patients.
Methods: we retrospectively enrolled 975 DTC patients treated with thyroidectomy+131-I remnant ablation showing undetectable S-Tg measured after 12 months follow-up. The availability of A-Tg and rh-TSH stimulated Tg (R-Tg) obtained 1 year later were considered as inclusion criteria. Patients with positive circulating Ab-Tg and/or histological dedifferentiation were excluded. Patients were subdivided in high and low risk of recurrence according to the criteria proposed by the European Thyroid Cancer Taskforce.
Results: Using rh-TSH test as gold standard, the NPV for A-Tg<10 µg/L was 98.5% in group A (low risk patients) and 95.5% in group B (high risk patients); it significantly raised to 99.2% in group A (p-value 0.03) and 99.3% in group B (p-value 0.02) when the association between A-Tg<10 µg/L and S-Tg<0.6 µg/L was considered. When we evaluated the whole population the negative predictive value was 97% for A-Tg<10 µg/L alone, raising to 99.3% when associated with S-Tg<0.6 µg/L (p-value<0.008).
Conclusion: our data confirmed the very high negative predictive value of the association between low levels of A-Tg and undetectable S-Tg in the early risk stratification of differentiated thyroid cancer patients, leading to avoid rh-TSH test with an important economic impact.
Ablation thyroglobulin; Suppressive thyroglobulin; Differentiated thyroid carcinoma; rh-TSH test
RAI: I-131 remnant ablation; DTC: Differentiated thyroid cancer; Tg: Thyroglobulin; THW: Thyroid hormone withdrawal; R-Tg: rh-TSH stimulated thyroglobulin; S-Tg: Suppressive thyroglobulin; A-Tg: Ablation thyroglobulin; NPV: Negative predictive value; PPV: Positive predictive value; WBS: 131-I Whole body scan; AbTg: Anti-Tg serum antibodies; DF: Disease free; PRD: Persistence/recurrence of disease; ROC: Receiver Operative Characteristic; CI: Confidence interval
Total or near-total thyroidectomy followed by I-131 remnant ablation (RAI) and thyroid hormone suppression therapy are usually considered as the standard approach for the initial treatment of differentiated thyroid carcinoma (DTC) [1-3]. Follow-up management is based on physical examination, neck ultrasonography and serum thyroglobulin assay (Tg). In particular, the undetectable stimulated Tg, which is measured during thyroid hormone withdrawal (THW) or after recombinant human TSH (rh-TSH) administration (R-Tg), is the main evidence of the patient’s recovery [2-4]. Unfortunately, this test appears quite expensive, considering that only a small percentage of patients with undetectable Tg on TSH suppression therapy (S-Tg) shows a response after TSH stimulation [5].
Despite the low risk of DTC-related death, a long-term follow-up is required since the rates of disease recurrence (up to 20% of patients) have been reported also many years after initial treatment [1]. In this context the number of patients who need monitoring for possible disease recurrence is very high and the early identification of patients at high risk of relapse is crucial in order to set a more aggressive treatment and closer follow-up. The initial staging is based on the clinicopathological system which has been elaborated by American Joint Cancer Committee/Union Internationale Contre le Cancer (AJCC/UICC)[6]. The main problem of this approach is that this system was designed to predict mortality [7], whereas a good follow-up program has to take into account the different risk of persistent or recurrent disease after the initial treatment. To overcome this limitation, the American Thyroid Association (ATA) [3] and the European Thyroid Association (ETA) [2] Consensus Statement subdivided thyroid cancer patients into different risk levels based on tumor-related parameters integrated with other clinical features, including histotype and age. Furthermore, these initial ATA/ETA risk estimates can be significantly improved through the assessment of patients’ response to initial therapy, thereby providing a dynamic risk evaluation as proposed by Tuttle et al. [8] and Castagna et al. [9].
In this context, some studies have observed that serum Tg levels measured just before RAI after total or near-total thyroidectomy (ATg) had the capability to predict the persistent/recurrent disease vs the disease-free status [10,11]. Particularly, a meta-analysis [12] including fifteen studies involving 3947 patients with DTC, showed 94% of negative predictive value (NPV) for A-Tg<10 µg/L. Moreover, a study [13] demonstrated that in patients at high risk of cancer there was a significant correlation between the increase of A-Tg levels and the risk of relapse, showing a positive predictive value (PPV) of 97% when patients have A-Tg>50 µg/L falling to 55% when 10<A-Tg<50 µg/L.
The aim of our study was to confirm these results in a wider population and to assess whether low A-Tg levels associated with undetectable S-Tg concentrations maintained a high NPV for recurrence of disease, leading to avoid rh-TSH test, also in high-risk DTC patients.
In this study we retrospectively evaluated 975 consecutive patients treated in the section of Nuclear Medicine at the Mauriziano Hospital of Turin from a total of about 2100 subjects treated with RAI from January 2001 to January 2011, in order to obtain a minimum follow up of 5 years. Since more than 80% of total RAI in Piedmont Region are performed in this Institution, this homogeneous population can be considered as representative of DTC behaviour during the last decade in a northwestern region of Italy.
All patients underwent: 1) total thyroidectomy (patients with clinically apparent nodal disease also underwent compartment-oriented neck dissection); 2) RAI (2960-3700 MBq) during THW 4-6 weeks after surgery; 3) 131-I whole body scan (WBS) 3-5 days after RAI therapy; 4) L-T4 suppressive therapy (serum TSH levels<0.2 µIU/mL); 4) rhTSH test 6-12 months after RAI. At least 2 years follow-up was available for each patient with mean follow-up of 5.4 years after thyroidectomy. Patients with positive anti-Tg serum antibodies (AbTg) and histological dedifferentiation during follow-up were excluded.
Serum Tg levels were measured after four weeks THW immediately before RAI (A-Tg) and after 3-6-12 months on L-T4 suppressive therapy (S-Tg). Then, patients received one injection of rh-TSH (0.9 mg i.m., Thyrogen®, Genzyme Corporation, Sanofi Company, Cambridge, MA) for two consecutive days; serum samples for TSH and Tg measurements were collected on days 0 (before first rh-TSH administration), 3 and 4. Neck ultrasonography was performed 3, 6 and 12 months after RAI.
Tg was measured using an immunoradiometric test (Cis Bio International, France) with a lower detection limit of 0.2 µg/L and a functional sensitivity of 0.7 µg/L. In our series only 6 patients showed R-Tg values of 0.7 µg/L and all of them resulted as disease free according to the criteria described below. Indeed, in order to make comparable our data with most literature reports, we considered 0.6 µg/L as the cutoff value between not measurable and measurable Tg levels, according to Mazzaferri et al. [4,15]. TSH and AbTg serum levels were measured using a chemiluminescent immunoassay (Access Immunoassay Systems, Beckman Coulter®, Inc, Brea, CA).
The patients were divided in high, low and very low risk according to the criteria proposed by the European Thyroid Cancer Taskforce [2] (Table 1). However, since the last group included only few patients as usually they did not undergo RAI treatment, the population was divided in group A (low risk+very low risk) and group B (high risk) as reported in table 2. In the whole population and in each subgroup the NPV value of A-Tg<10 µg/L alone and associated with undetectable S-Tg was evaluated.

Table 1: Subdivision of patients into three risk categories according to European Thyroid Cancer Taskforce [2].
Patients with negative neck ultrasonography and R-Tg<0.6 µg/L were considered as disease free (DF). Patients with R-Tg>2 µg/L, S-Tg>0.6 µg/L or with imaging evidence of recurrence were classified as persistence/ recurrence of disease (PRD) and underwent further treatments, as surgery and/or RAI. Patients with R-Tg between 0.6-2 µg/L were considered as equivocal and they were reclassified according to the subsequent follow-up outcome. In particular, they were submitted to an additional Tg stimulation test with rh-TSH 12 months later. If serum Tg became undetectable or remained at the same values of the previous test the patient was considered as DF, being most probably due to normal thyroid remnant [16].
Post-therapy WBS was performed in all population, while only in patients with suspected PRD was conducted a further diagnostic WBS to evidence extra-thyroid uptake.
Commonly used statistical methods have been applied to describe the data (means, standard deviation, range, frequencies, percentage). The cut-off levels for A-Tg were selected by Receiver Operative Characteristic (ROC) curve analysis. The statistical significance of differences between qualitative variables was assessed with Chi square (χ2) test, with Yates correction when necessary, or the Fisher’s exact test, while it was evaluated with Wilcoxon test between quantitative variables. Sensitivity, specificity, NPV and PPV were calculated for A-Tg alone and the association of A-Tg and S-Tg using the rh-TSH test as gold standard and the confidence interval (CI) was set at 95%.
All data analyses were performed by the SAS package for Personal Computer (SAS Inc. V 8.2, Cary, NC).
The study cohort was made up of 732 (75.1%) female and 243 (24.9%) male patients. The mean age at diagnosis was 49.1 ± 14.7 years (range 11- 86 years). The histopatological types consisted of 766 (78.6%) papillary carcinoma and 209 (21.4%) follicular carcinoma, including 42 Hürthle cell carcinoma. According to epidemiological data of DTC [17-19], males had a lower incidence but they showed a more aggressive histological phenotype and, consequently, resulted in an increased risk of PRD. Clinical and outcome data of patients are reported in table 2.
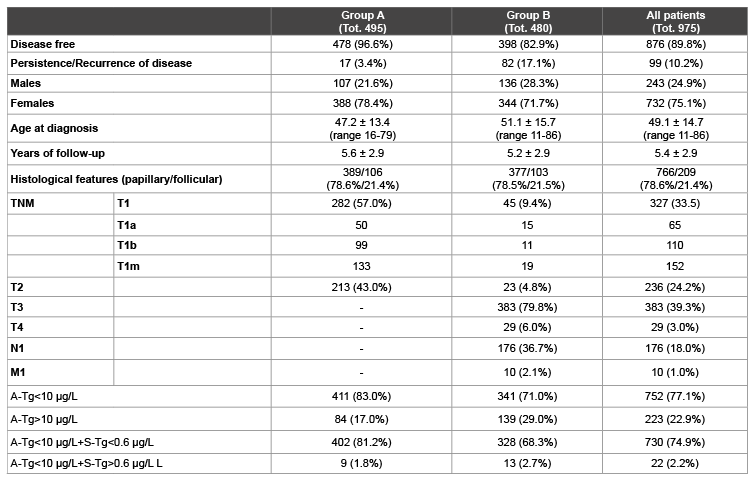
Table 2: Clinical and pathological patient’s features in group A (very low risk + low risk), group B (high risk) and whole population. TNM classification system according to American Joint Committee on Cancer (AJCC) [6]. A-Tg: Tg measured just before RAI after total or near-total thyroidectomy. S-Tg: Tg on TSH suppression therapy.
As reported in literature [12], we used the threshold of A-Tg<10 µg/L (sensitivity 78.4%, specificity 83.8%, NPV 96.9%), which was similar to the A-Tg cut-off of 12 µg/L suggested by ROC curve analysis performed on our population (figure 1) (sensitivity 81.2%, specificity 79.9%, NPV 97.3%).
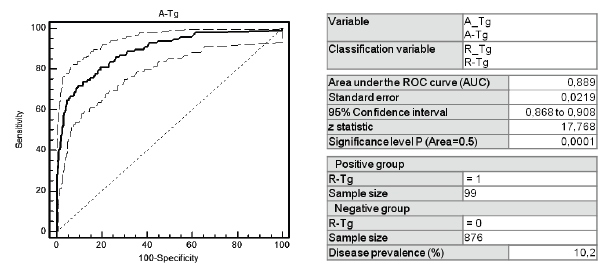
Figure 1: ROC curve to select the optimal threshold of A-Tg values in our population. A-Tg: Tg measured just before RAI after total or near-total thyroidectomy. R-Tg: Tg measured after rh-TSH somministration.
Post-therapy WBS showed 843/975 (86.5%) and 119/975 (12,2%) patients with thyroid and extra-thyroid uptake respectively. All data about initial WBS performed and the subsequent follow-up were described in table 3.

Table 3: Post-therapy WBS uptake and the consequence follow-up in the all population.
In the whole population, 876/975 (89.8%) patients were defined as DF and 99/975 (10.2%) as PRD (table 2).
Overall, 752/975 (77.1%) showed A-Tg<10 µg/L (table 4); among them 729/752 (96.9%) were DF and 23/752 (3.1%) were PRD. Based on these data, the NPV of A-Tg alone was 96.9%, with sensitivity of 78.4% and specificity of 83.8% (table 5).
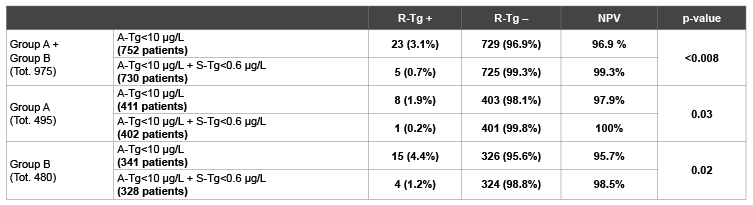
Table 4: Negative Predictive Value (NPV) of A-Tg<10 µg/L alone and associated with undetectable S-Tg in the whole population, in group A and in group B. A-Tg: Tg measured just before RAI after total or near-total thyroidectomy. S-Tg: Tg on TSH suppression therapy. R-Tg: Tg measured after rh-TSH somministration.
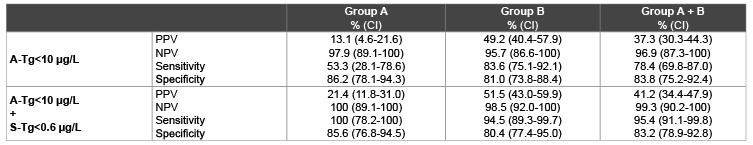
Table 5: Sensitivity, specificity, Positive Predictive Value (PPV) and Negative Predictive Value (NPV) in group A, B and whole population. A-Tg: Tg measured just before RAI after total or near-total thyroidectomy. S-Tg: Tg on TSH suppression therapy.
Furthermore, 730/975 (74.9%) patients showed A-Tg<10 µg/L and S-Tg<0.6 µg/L, of which 725/730 (99.3%) patients resulted as DF and 5/730 (0.7%) were PRD. The distribution of patients is reported in figure 2. Therefore, when A-Tg<10 µg/L was associated with S-Tg<0.6 µg/L the NPV raised to 99.3% (p-value<0.008), with a simultaneous increase in sensitivity (78.4% vs 95.4%), maintaining the same specificity (83.8% vs 83.2%).
495/975 subjects were included in group A (table 2); 478/495 (96.6%) were defined as DF based on R-Tg<0.6 µg/L and negative neck ultrasonography.
411/495 (83.0%) showed A-Tg<10 µg/L (table 4); among them 403/411 (98.1%) were DF and 8/411 (1.9%) were PRD. In this group of patients the NPV of A-Tg alone was 97.9%.
Moreover, 402/495 (81.2%) patients showed A-Tg<10 µg/L and S-Tg<0.6 µg/L. In these 402 patients the NPV, sensitivity and specificity of the association of A-Tg<10 µg/L and S-Tg<0.6 µg/L was 100% (p-value 0.03), 100% and 85.6% respectively (Table 5).
480 subjects were included in group B (table 2); 398/480 patients (82.9%) were defined as DF based on R-Tg<0.6 µg/L and negative neck ultrasonography.
Overall, 341/480 (71.0%) showed A-Tg<10 µg/L (table 4); among them the NPV of A-Tg<10 µg/L was 95.7%. In this group, the association of A-Tg<10 µg/L and S-Tg<0.6 µg/L showed a NPV of 98.5% (p-value 0.02), with a sensitivity of 94.5% and a specificity of 80.4% (Tables 4 and 5).
The follow-up of patients with DTC is mostly based on serum Tg measurement for identifying patients with persistent or recurrent disease. Detectable S-Tg levels have been recognized as a good marker of DTC recurrence after initial treatment, whereas R-Tg is probably the most important means to define remission of disease [2-3].
Recent studies [10-14] assessed the predictive value of A-Tg showing a significant correlation between A-Tg levels and the values of stimulated Tg after rh-TSH. In particular, a meta-analysis [12] involving nearly 4000 patients, confirmed that A-Tg levels can be a useful negative predictor of persistent or recurrent DTC, defining a cut-off value of 10 µg/L as optimal sensitivity and specificity. About 70% of patients had Tg levels below this threshold and the overall NPV was 94.2%.
Our previous study [14] extended this observation and showed that low risk DTC patients with A-Tg levels<10 µg/L combined with undetectable S-Tg had a NPV for disease recurrence of 97.1%, rising to 100% when 2 years follow-up was taken into account; in particular none of patients with A-Tg<10 µg/L showed a significant response to rh-TSH (R-Tg>2 µg/L).
In the present study, we retrospectively evaluated the prognostic significance of A-Tg, alone and associated with S-Tg, both in each class of risk according to European Thyroid Cancer Taskforce criteria [2], and in the whole population of DTC patients. Our data showed that A-Tg<10 µg/L has a NPV of 96.9% in the whole population, according to the data reported in literature [2]. When the A-Tg was associated with undetectable S-Tg levels, the NPV significantly raised to 99.3% (p-value<0.008) with a considerable sensitivity improvement (78.4% vs 95.4%) maintaining a similar specificity (83.8% vs 83.2%). Moreover, these results were reproduced in both risk groups, when the patients were subdivided in two groups based on different risk of persistent/recurrent disease. In group A (low risk+very low risk of recurrence) A-Tg associated with S-Tg levels showed a NPV of 100%, while in group B (high risk of recurrence) it resulted slightly lower (98.5%) compared to the whole population, however confirming itself as a reliable prognostic index.
According to these data, we hypothesize that it could be possible for a group of patients to avoid the rh-TSH test during follow-up based on A-Tg<10 µg/L and S-Tg<0.6 µg/L values. In particular, in our population 730/876 (83.3%) DF patients could have avoided the expensive Tg stimulation test (figure 2).
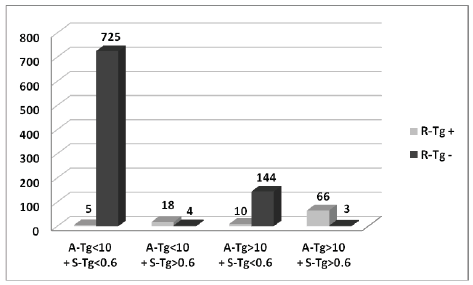
Figure 2: Patients distribution based on A-Tg and S-Tg values in the whole population. A-Tg: Tg measured just before RAI after total or neartotal thyroidectomy. S-Tg: Tg on TSH suppression therapy.
Since in our study 478/495 (96.6%) of very low risk and low risk patients (group A) and 398/480 (82.9%) of high risk patients (group B) resulted as DF, we believe that risk stratification according to European Thyroid Cancer Taskforce [2] assigns too many subjects to high risk group without a really PRD evidence during follow-up. This concept is also reported by Castagna et al. [9] showing that 53% of high risk patients can be subsequently reclassified as low risk after 8-12 months follow-up from the initial treatment.
Altogether, the association between A-Tg and S-Tg can be considered an important completing factor for initial risk stratification of DTC patients, beyond clinical and histological parameters. As a matter of fact, the association between A-Tg<10 µg/L and S-Tg<0.6 µg/L may correctly identify 77.1% (725/975) of patients as DF, regardless of the ETA risk stratification. The combination of these two parameters had only 5/975 (0.5%) false negative; these cases may be early detected as relapsed/ recurrent disease during the following one-year follow-up on the basis of the increasing value of S-Tg and/or ultrasound evaluation.
In our study, A-Tg>10 µg/L was considered a criterion for the early classification of patients at high risk of PRD. As illustrated in figure 2, 223/975 (22.9%) patients showed A-Tg>10 µg/L. Among them 147/223 (65.9%) patients were DF and 76/223 (34.1%) patients were PRD.
A-Tg>10 µg/L values can identify only 76.8% (76/99) of patients with PRD compared to the percentage obtained by ETA risk classification system (82.8%). However, when we considered A-Tg>10 µg/L combined with S-Tg>0.6 µg/L, we correctly identified 66/69 (95.5%) patients at higher risk of recurrence, thus obtaining a good initial risk stratification for PRD, which led to a more accurate therapeutic choice planning.
A limitation of this study is the employment of serum Tg assay with lower detection limit of 0.2 µg/L and functional sensitivity of 0.7 µg/L. However it has to be considered that higher sensitive assays have been widely used in the clinical practice only in the last years. Nevertheless we choose to begin the enrollement in 2001 in order to obtain a large population with a long time of follow up. Another limit is represented by the lack of data about remnant entity, which presented certain variability since the surgery was performed in various centers.
On the other hand, an advantage of this study is the fact that our Institution performs more than 80% of total RAI in Piedmont Region, allowing to consider this population as homogeneous and representative of DTC behaviour in the last 10 years in this area.
In conclusion, our data confirm the usefulness of the association between A-Tg and S-Tg in the early risk stratification of DTC patients, leading to avoid rh-TSH test in a wide number of subjects and to a more accurately therapeutic choice planning, with a remarkable economic impact. We suggest that the association between A-Tg<10 µg/L and S-Tg<0.6 µg/L combined to negative neck ultrasonography can be considered as sufficient parameters for classifying DTC patients as DF.
FA and FB contributed equally to this work.
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
The Authors would like to thank Dr Claudia Cavallari for helpful suggestions and comments.
Download Provisional PDF Here
Article Type: Research Article
Citation: Arecco F, Bardesono F, Corvisieri S, Messuti I, Puligheddu B, et al. (2016) Is it Possible to avoid rh-TSH test in Patients with Differentiated Thyroid Carcinoma by Using the Association between Ablation and Suppressive Thyroglobulin? Int J Endocrinol Metab Disord 2(2): doi http://dx.doi.org/10.16966/2380- 548X.126
Copyright: © 2016 Arecco F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access