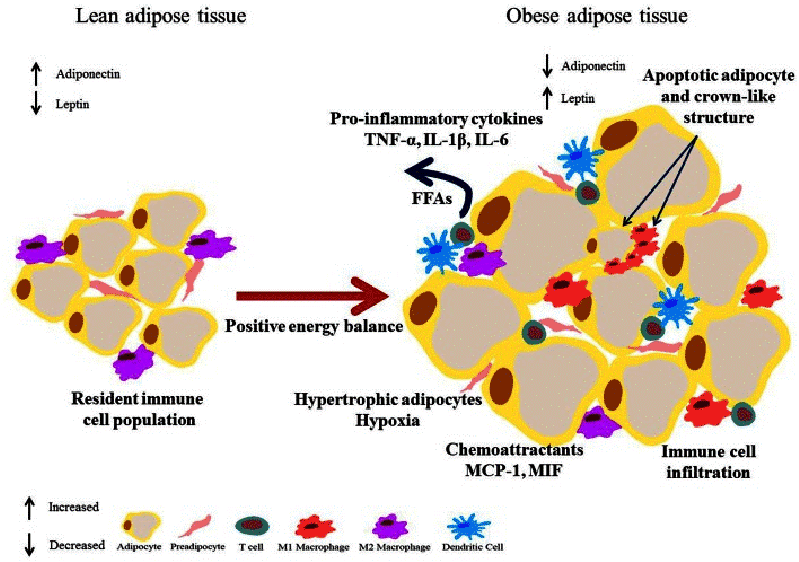
Figure 1: Large energy intake (hyperfagia) combined with sedentary behavior [14]

Govers E1* Slof E2 Verkoelen H3 Ten Hoor-Aukema NM4 Knowledge Centre for Dietitians for Prevention Management of Overweight Obesity (KDOO)
1Chair to ESDN Obesity of the European Federation of Associations of Dietitians (EFAD), Primary Care Dietitian to Amstelring, Institution for Primary Care, The Netherlands*Corresponding author: Elisabeth Govers, Cornelis van Alkemadestraat 16, 1065 AC Amsterdam, The Netherlands, E-mail: e.govers112@upcmail.nl
The successes of interventions to obtain weight loss and prevent relapse are limited. Moreover, comorbidities like type 2 diabetes mellitus, hypertension, hypercholesterolemia, hypertriglyceridemia and gout, have so far been treated as separate diseases, although mounting evidence shows that these morbidities are consequences of the failing metabolism due to insulin resistance. Weight loss, in other words treating obesity, improves comorbidities and improves quality of life. Treatment of obesity and its comorbidities is a multidisciplinary matter. It can be done in primary care. It should be widely recognized that a low carbohydrate diet and exercise are the two main aspects of treatment that lead to the desired result: considerable weight loss and diminishment of comorbidities, visible through improvement of blood parameters and improved quality of life. Because of the complexity of the diet a large role in management is fit for dietitians, supported by psychologists, physiotherapists and exercise trainers. Family physicians and nurse practioners need to be aware of the important role diet and lifestyle play. In insulin resistance medication is not the preferred treatment; it should be avoided as much as possible. By accepting this challenge in primary care, health professionals can change the prevalence and consequences of obesity and its comorbidities, thus reducing health care costs considerably. Persons that are insulin resistant may regain their health through these measures. They will always stay insulin resistant to a certain extent, and cannot eat normal quantities of carbohydrates that are commonly used and advised in general dietary guidelines.
Obesity; Insulin resistance; Comorbidities; Type 2 diabetes; Thyroid gland; Fasting insulin; Low carbohydrate diet; Vitamin D; Physical exercise; Sleep
Insulin resistance is a condition that arises when a person gains weight to an extend that the normal (subcutaneous) fatty tissue is overfilled and fat accumulation takes place in the abdomen, the liver, the muscles and in a later stadium in the brain, arteries and intestine. The majority of this fatty tissue is stored in the abdomen, in between the organs. This visceral fat develops, contrary to subcutaneous fat, into an active endocrine organ. The adipocytes secrete an abundance of adipokines, which alter the metabolism. Major problems resulting from insulin resistance are elevation of blood pressure and elevated insulin levels, leading to cardiovascular disease and type 2 diabetes, respectively. Insulin resistance is not diagnosed routinely by measuring fasting insulin levels; measuring waist circumference is commonly used as the diagnostic tool, leading to missed diagnosis in many cases. Persons who are insulin resistant have trouble losing weight on a diet with a normal percentage of carbohydrates.
Obesity is a major health problem worldwide. The prevalence has more than doubled since 1980. In 2014 more than 1.9 billion adults, which means that 39% of 18 years and older were overweight; 600 million were obese (13%) [1] leading to comorbidities such as coronary heart disease, hypertension and type 2 diabetes.
The successes of interventions to obtain weight loss and prevent relapse are limited [2]. Moreover, comorbidities like type 2 diabetes mellitus, hypertension, hypercholesterolemia, hypertriglyceridemia and gout, have so far been treated as separate diseases, although mounting evidence shows that these morbidities are consequences of the failing metabolism due to insulin resistance (IR). Weight loss, in other words treating obesity, improves comorbidities and improves quality of life [2-14].
Underestimating the importance of insulin resistance has reduced obesity to a health problem characterized by an overload of kilos instead of a metabolic problem. Ignoring the metabolic component of obesity has also lead to ineffective treatment of the comorbidities.
In this guideline the mechanisms underlying insulin resistance are explained, as well as the individualized dietary treatment leading to sustainable weight loss, improved insulin sensibility reducing comorbidities and improved quality of life.
The insulin resistance syndrome (IRS) is also known as metabolic syndrome and syndrome X [11]. In the last decade it became clear that accumulation of the visceral fat leads to impaired function of insulin in the organs and muscles. In this process the amount of fatty tissue is important, as well as the location of the free fat mass.
Figure 1 makes clear how a large energy intake (hyperfagia) combined with sedentary behavior leads to a positive energy balance, causing overfilling of the adipocytes. Fat storage normally takes place in the fat depots in de the body, mainly under the skin. When an abundance of calories is accumulated by chronic caloric overfeeding the fat depots will get overfilled and fat storage will also take place in the abdomen. This abdominal or visceral fat is stored between the organs in the abdomen. There it develops into an active endocrine organ. In the adipocytes the bloodstream decreases, leading to low oxygen levels in the cells (hypoxia), followed by a low grade inflammation and infiltration of macrophages that enhance insulin resistance. As a result the tissue produces chronically elevated levels of adipocytokines, for instance tumor necrosis factor-alpha (TNF-a) en leptin contribute to the development of IRS. In this way a vicious circle is created: the chronic overproduction of adipocytokines leads to more IRS, which on its turn leads to more visceral fatty tissue, which causes synthesis of more adipocytokines, that have an influence on processes within the adipocytes and on several organs, e.g. the pancreas, the liver, and metabolic processes, e.g. blood pressure, blood glucose, blood lipids and purines thus leading to comorbidities. For example: leptin and tumor necrosis factor-alpha play a role in pro-inflammatory immune reactions en oxidative stress. This process influences atherosclerotic changes in the arteries [3].

Figure 1: Large energy intake (hyperfagia) combined with sedentary behavior [14]
Other aspects of metabolic stress are: adiponectin levels decrease, a factor that leads to hypertension. Leptin increases. In obesity, a decreased sensitivity to leptin occurs, resulting in an inability to detect satiety despite high energy stores and high leptin levels [4]. Food choices have a strong influence on development and grade of insulin resistance: metaflammation in fat cells occurs faster when people consume large quantities of carbohydrates and saturated fat. Cafeteria food and other fast food leads faster to insulin resistance than traditionally cooked food rich in carbohydrates and saturated fat [6].
Overweight leads in 60-85% to insulin resistance followed by comorbidities. The longer a person is obese and the higher the BMI, the stronger the insulin resistance will be [14,15]. There is a strong genetic component in the prevalence of IRS, because fat distribution, obesity and type 2 diabetes mellitus are hereditary conditions to a large extend [16,17]. The use of anti-depressivants and anti-psychotic medication enhances insulin resistance [18,19].
The foremost symptom of aroused insulin levels however is overweight with a large waist circumference. If the waist is 80% or more of the hip circumference, it is likely that the patient is insulin resistant [20].
Hyperinsulinaemia once it is present, leads to: further weight gain, especially in the abdomen and around the waist. It can be accompanied by normal or elevated fasting glucose levels, hypertension, dyslipidemia (HDL goes down, LDL, total cholesterol and triglycerides go up), nonalcoholic fatty liver disease (NAFLD) sleep apnea, osteo arthritis, elevated purine levels and gout, poly cystic ovarian syndrome (PCOS), elevated estrogen levels, and lowered testosterone levels. To high fasting glucose levels are a sign of insulin resistance, caused by the nightly gluconeogenesis from the liver, rather than a sign of type 2 diabetes, especially when HBA1C (GlyHb) is normal. Other symptoms of IR are fatigue, emotional instability, chronic infections and infertility. Too high carbohydrate levels in the duodenum enhance the activity of the mast cells, thus promoting allergic reactions, the growth of fungi and the parasite Blastocystis Hominis [21].
After a longer period of time, the pancreas fails in meeting insulin needs after meals, leading to impaired glucose tolerance, and finally to type 2 diabetes. Insulin resistance has also been linked to the prevalence of breast, prostate, and colon cancers. For prostate cancer it has been shown that hyperinsulinaemia acts on the liver to increase production of insulin-like growth factor-I (IGF-I), a factor known to stimulate tumor growth and block apoptosis. Insulin resistance leads to over activity of mast cells in intestine, lung, and skin, causing allergic reactions. In children and adolescents, HOMA estimated insulin resistance values were significantly associated with positive skin tests and allergic asthma diagnosis (Table 1). There was a strong relationship between a large waist circumference and pulmonic function. Patients with mild stages of COPD often have obesity and insulin resistance. Patients with COPD and metabolic syndrome have increased risk of morbidity and mortality due to cardiovascular disease. Growing evidence supports the concept that insulin resistance is important in the pathogenesis of cognitive impairment and neurodegeneration. Insulin plays a profound role in cognitive function. Impaired insulin signaling in the advancement of cognitive dysfunction is relevant to the pathophysiologic mechanisms of cognitive impairment and the risk of developing dementia. The relationship between sleep apnea and metabolic syndrome is well known [4,21].
In post-menopausal women the thyroid gland can show deviating values enlarging the risk of developing metabolic syndrome. There is a strong relationship between metabolic syndrome and elevated TSH, free T3 and free T4 values and the HOMA-IR [22]. In obese men and women metabolic syndrome was seen in 58% with significantly higher fasting glucose, triglycerides, free T4, systolic and diastolic blood pressure, significantly lower HDL-cholesterol and free T3/FT4 ratio than those without metabolic syndrome. With the exception of HDL-cholesterol all values correlated with T3, free T4, and the free-T3/free-T4 ratio. Free T4 levels were associated with obesity and metabolic syndrome, independent of insulin resistance, whereas T3 levels were associated with insulin resistance and metabolic syndrome. This association can be explained by the compensating mechanism of T3 and free T4 on energy expenditure and thermogenesis in obese individuals [23] (Table 2). A strong accumulation of visceral fat is associated with increased levels of free T3 and TSH in the serum, independent of insulin sensitivity, metabolic parameters and blood pressure. This indicates that the body adjusts the thermogenesis to the increase of fat mass, and that the balance between TSH and free T3 and T4 in the serum is disturbed. No correlation between free T 4 and waist circumference was seen. It is often seen that although obese individuals have trouble losing weight T4 values are often normal. T4 levels may possibly not be a good measurement for thyroid function in obese persons [24].
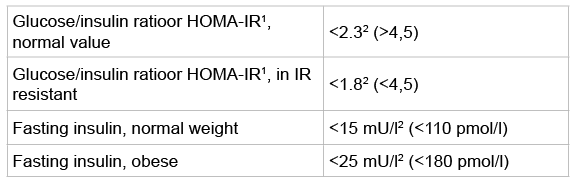
Table 1: Blood parameters to establish insulin resistance [35,36]
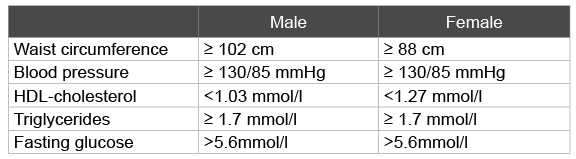
Table 2: Blood parameters to establish metabolic syndrome
Vitamin D status in overweight and obese people that are insulin resistant or pre-diabetic is often too low [25-33]. The 25(OH)D serum levels can be significantly negatively correlated with BMI (P<0.01), waist circumference (P<0.05), fasting insulin (P<0.01), HOMA(IR) (P<0.01), triglycerides (P<0.01), CRP (P<0.01), C3 (P<0.05), and C4 (P<0.05) in healthy male and female with overweight and obesity. Multiple regression analysis with 25(OH)D as the dependent variable showed that insulin and HOMA(IR) both had a significantly independent association with 25(OH)D levels [34].
Insulin sensitivity is best measured through the fasting glucose/insulin ratio (HOMA-IR). When the ratio is <1.8 (SI value/4.5 metric value) the patient is insulin resistant [35-37] (Table 1). To get an insight in values in primary care practice a medical doctor investigated insulin sensitivity in her own patients. She measured fasting and non-fasting glucose and insulin values in at random selected patients at a fasting state, and after a breakfast and lunch with 60 gram carbohydrates. Breakfast had 60 grams carbohydrates with 54% mono- en disaccharides; lunch contained 60 grams of carbohydrates with73% of mono- and disaccharides (Table 3) [19].
At the start of the treatment the dietitian makes a dietetic diagnosis [38].
This is an assessment of:
Explanation about the physiological changes in the patient’s body through visceral fat and IR gives an insight in the causes of overweight, and the reasons why weight loss in the past has not been successful in the long term. It forms the basis for lifestyle changes and self-management by the patient. It is important to formulate the patient’s own objectives as well as the objectives of the dietary treatment, because these may be different. Patients have their own thoughts about their health and what they want to achieve, which are sometimes not realistic, e.g. the wish to lose 30 kilos in six months; and sometimes show a lack of commitment, e.g. when a patient does not want to lose weight when he is developing type 2 diabetes. The challenge for the dietitian is to motivate the patient to feel responsible for his own health; to feel confident that he can make a few changes that mean a big difference, and to keep him interested whilst he has a busy work environment and social life. It is very important to keep focus on weight loss, because weight loss is the key to improvement of comorbidities, and long-term quality of life. Dietary management aimed at minor weight loss may lead to patient satisfaction short term but may not lead to improved physiological and metabolic health [39].
Dietary treatment aims at improving and normalizing metabolic and vascular health and implies:
Decide together with the patient, based on the dietetic diagnosis, the strategy for the treatment, which grade of carbohydrate restriction is indicated, what the protein content of the diet should be and frequency and duration of physical exercise, to support the diet.
Under Dutch law a diet is a nutritional treatment that differs from general dietary guidelines for a medical reason. Insulin resistance is a medical condition that requires a different proposition of the macronutrients. A diet with the normal advice to obtain 50 energy percent from carbohydrates, as general guidelines worldwide do, is not fit for the patient with insulin resistance, because these patients will not lose weight. Such a diet will not be effective and sometimes even counter-productive [45,46]. Self-help diets and commercial or internet based diets do not meet the requirements for diets on medical indication needed for patients with insulin resistance.
The explanation why patients with IR do no lose weight on normally composed diets is that the insulin level at fasting state already is too high (Table 2). Large quantities of carbohydrates stimulate the release of insulin even more; insulin promotes lipogenesis. Through the high insulin levels the release of growth hormone is inhibited. Growth hormone promotes lipolysis. High insulin levels thus prevent lipolysis, and therefore prevent weight loss. This is why the diet is aimed at reducing the secretion of insulin through diet to a minimum, to promote lipolysis and consequently weight loss.
Dependent on the grade of insulin resistance and based on the dietary history the carbohydrate content of the diet is decreased. A low carbohydrate diet is high in protein and fat and contains optimal micronutrients and fibre [46].
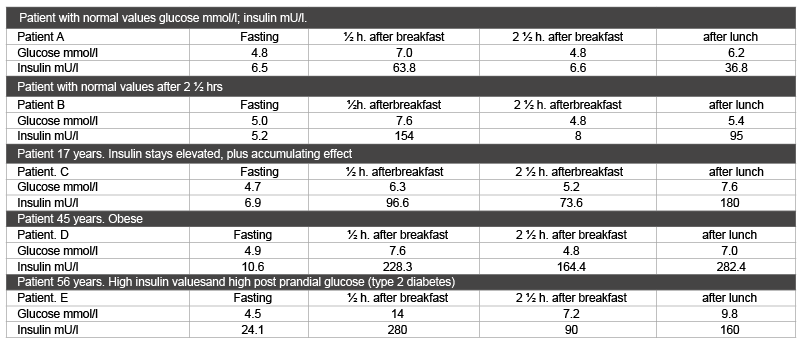
Table 3: Glucose and insulin values in at random selected patients.
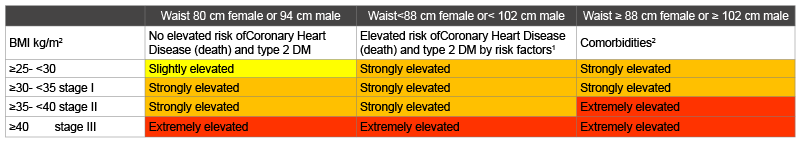
Table 4: Assessment of health risk [39]
1
10-year risk of death of CHD> 5% orimpaired fasting glucose
2
having type 2 DM, CHD, sleep apnoea, osteo arthritis.
The objectives of the dietary management for insulin resistance are:
Both body weight and fat free mass (FFM) are important determinants of the basal metabolism. Organs and muscle have high energy expenditure and fat mass uses less energy. The percentage muscle or body fat influences basal metabolism: individuals with a high fat percentage have a lower basal metabolism than those with a lower fat percentage.
A person of 95 kg with a low fat percentage has more muscle mass (FFM), than someone with the same weight and a high fat percentage, and therefore has a higher basal metabolism. As Table 5 shows two persons with the same weight, one with 20% fat mass and one with more than 30% fat the first has an energy expenditure of 400 calories more [47,48]. This difference needs to be taken into account when calculating energy expenditure for a patient. In insulin resistance the energy expenditure must also be taken into account: weight loss never occurs when there is no energy deficit. Generally a deficit of 600 calories forces the body to metabolize fatty tissue. As mentioned before in insulin resistance the low carbohydrate approach is necessary to start lipolysis.
Fat mass cannot correctly be measured with waist circumference: this measurement indicates abdominal fat, but gives no insight in visceral fat and percentage fat free mass. This is best done with a four-point impedance meter. A large waist circumference however is an easy way to establish insulin resistance.
The difference in basal metabolism between men and women can also be explained by differences in fat percentage: women have averagely 10 per cent more fat mass than men with the same length, weight and age. At the rise of age this difference diminishes [49].
A carbohydrate restriction has a greater effect on lowering serum glucose values than a caloric restriction and should be the first choice in treatment of type 2 diabetes [50]. Table 6 gives an outline of the carbohydrate restrictions for patients with different profiles.
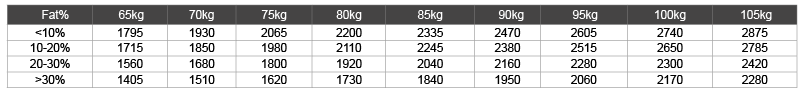
Table 5: Average basal metabolism per 24 hours in persons aged 20-40 according to body fat percentage
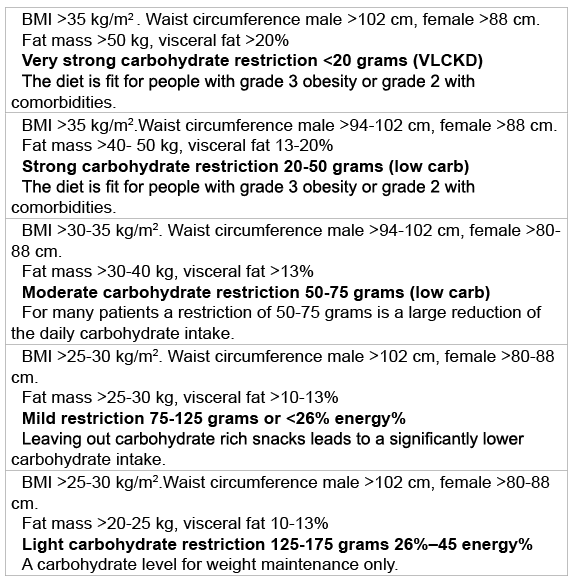
Table 6: Level of carbohydrate restriction related to BMI and waist circumference
Fat in the diet plays an important role in both taste and satiation, thus leading to more compliance. One of the main advantages of a low carbohydrate diet is that there is no fat restriction. Dependent of the carbohydrate restriction fat can contribute 35-50 energy % to the diet. Fear of too high consumption of fat is overestimated [55,56]. However, one needs to consider total energy intake to make weight loss possible. Therefore, fat cannot be added to the diet unlimitedly. If weight loss in a low carbohydrate diet is not achieved, it is advised to reconsider the fat intake in the diet.
Up to a few years ago the dominant approach in weight loss management was that calorie restriction was most effective. The result was, besides the fact that patients were hungry part of the time, that muscle mass declined. A protein intake of 1 to 1,5 grams per kilo body weight, reducing carbohydrate intake at the same time has a beneficial effect on body composition. Patients lose body fat, whereas fat free mass (muscle, bones, organs) will be preserved. A high protein diet with a firmly reduced carbohydrate intake has proven most effective to maintain muscle mass and lose fat mass [67].
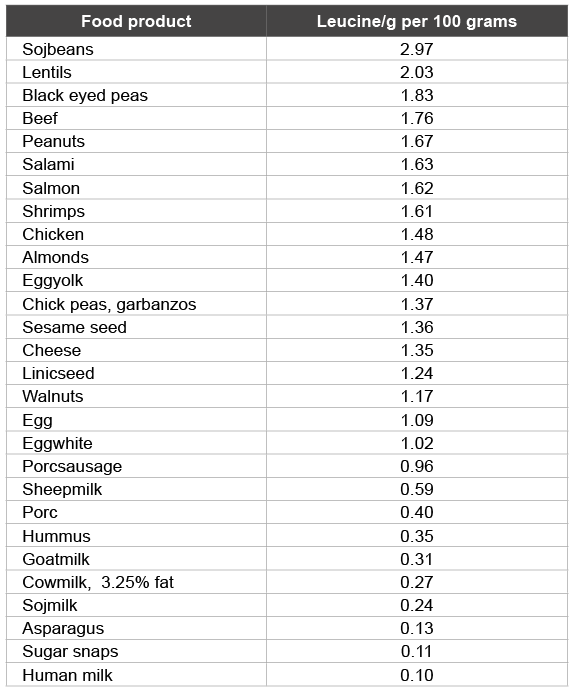
Table 7: Leucine in uncooked and unprocessed food
High protein meals give more satiation. Because appetite is postponed for hours after a protein-rich meal, it is easier not to eat in between meals and uphold the diet [68]. If the patient is able to control his appetite this is a remedy against relapse. A diet rich in protein and low in carbohydrates is good for long-term weight maintenance [69].
Fibre intake is an important anamnestic item in obesity management: constipation leads to bad compliance.
The objectives for suppletion are prevention of deficiencies, improvement of micronutrient status and restoration of the insulin sensitivity. Many patients with IR have eaten unhealthy food for a long time and therefore do not have an optimal nutrient status. A low carbohydrate diet is insufficient in vitamin B1, selenium, iodine, magnesium and manganese. Suppletion is therefore essential. Usually 1x the ADA will do. It is also the most sensible choice because the micronutrients in these products are balanced (Table 8).
Oxidative stress contributes to IR and is associated with oxidative damage. Anti-oxidants may have a positive influence on prevention of further disfunctioning of the glucose metabolism [66,73].
The level of physical activity is a major part of energy expenditure. The PAL-value (PAL=Physical Activity Level) is the factor with which the basal metabolism must be multiplied to calculate the 24-hour energy expenditure. The average PAL-value varies between 1,0 in sedentary to 2,4 in very active persons with intensive sports or heavy physical labor. A sedentary person weighing 105 kg and having >30% fat mass needs per 24 hours: 2280 × 1,0=2280 kcal. In obesity the required level of activity is one hour moderately intensive per day.
Physical exercise is essential to reduce IR and to achieve weight loss. Constant exercise causes strong anti-inflammatory effects, probably because of the influence of exercise on the immune system, and through the reduction of visceral fat [74-76]. Exercise also causes a reduced release of pro-inflammatory cytokines and chemokines from the adipocytes [77].
Endurance exercise leads to reduce induction of pro-inflammatory signaling and obesity [78-81]. It also diminishes the infiltration of macrophages in the fatty tissue and promotes anti-inflammatory immune cellphenotype [82]. During training muscle cells probably release many anti-inflammatory cytokines.
Walking, swimming and cycling are advised, one hour per day. In the starting phase every other day to prevent over-training, good results were seen in patients that exercised 150 minutes moderately intensive per week [44]. Patients with very little muscle mass, measured with a four-point impedance meter see their muscle mass improve with power lifting.

Table 8: Vitamins and minerals
Sleep deprivation leads to insulin resistance. Sleep plays a key role in homeostasis of the glucose metabolism. Normally glucose metabolism has a daily pattern with intra-individual variations in glucose tolerance: glucose expenditure is highest in waking state and lowest during NONREM sleep. Sleep deprivation leads to increase of the glucose production with 22%, suggesting hepatic insulin resistance. The speed of glucose turnover was reduced with 20 percent, an indication of reduced peripheral sensitivity for insulin. Short night rest caused as well an elevated plasma level of non-estherized fatty acids [83].
So far, type 2 diabetes has been considered as a separate disease, accompanied by obesity. Insulin resistance is rarely taken into account. We know now that insulin resistance is the forebode of type 2 diabetes, and the cause of it. We therefore need a different approach to management of type 2 diabetes, where insulin levels are the central issue to be dealt with, not glucose levels. As a result of that new approach we should treat patients with low carbohydrate diets and refrain from medication as long as possible. Elevated fasting glucose levels are not a sign of pancreas insufficiency, but of insulin resistance. Blood glucose lowering medication is treating the symptoms instead of the cause. After having type 2 diabetes for many years, obese patients may not be sensitive to oral medication and insulin any more. Increasing the insulin load does not lead to better diabetes management, and often not even to an acceptable HBA1C level. A patient with type 2 DM and insulin injections may use too much insulin because of a high endogenic insulin level, even after long-term use.
In case of a low carbohydrate diet, sulfoneum derivates and insulin need to be lowered based on glucose values as a result of the diet, with the aim to enhance weight loss. Weight loss leads to less need of medication [84].
At the start of a strong carbohydrate restriction (Table 6) the dosage of sulfoneum derivates and insulin can be put in half. This decision is made in close cooperation with the physician and nurse practitioner.
Treatment of obesity and its comorbidities is a multidisciplinary matter. It can be done in primary care. It should be widely recognized that a low carbohydrate diet and exercise are the two main aspects of treatment that lead to the desired result: considerable weight loss and diminishment of comorbidities, visible through improvement of blood parameters. Because of the complexity of the diet a large role in management is fit for dietitians, supported by psychologists and physiotherapists and exercise trainers. Family physicians and nurse practioners need to be aware of the important role diet and lifestyle play. In insulin resistance medication is not the preferred treatment, it should be avoided as much as possible. A patient with hypertension for example should be treated for the cause, and enter a lifestyle treatment instead of entering a stepup medication protocol. Accepting this challenge in primary care, health professionals can change the prevalence and management of obesity and its comorbidities, thus reducing health care costs considerably.
Insulin resistance is a serious condition caused by a too large fat mass, especially when located in the abdomen, leading to metabolic disease, such as hypertension, glucose intolerance, dyslipidaemia, type 2 diabetes and cardio vascular disease. It is best diagnosed by measuring fasting glucose levels. Measuring waist circumference and calculating BMI are additional instruments. Management should focus on weight loss through a low carbohydrate diet, with sufficient fat, protein, vitamins and minerals. Exercise is an essential part of management and relapse prevention. Persons that are insulin resistant may regain their health through these measures. They will always stay insulin resistant to a certain extent, and cannot eat normal quantities of carbohydrates that are commonly used and advised in general dietary guidelines.
Elisabeth Govers works as a primary care dietitian and has no conflict of interest.
Nienke ten Hoor works as a primary care dietitian and has no conflict of interest.
Erica Slof works as a primary care dietitian and also works for Atkins.
Harriet Verkoelen works as a primary care dietitian and a trainer and has no conflict of interest.
The authors wish to thank all dietitians, physicians and nurses they have worked with in management of obesity and its comorbidities. They wish to thank their patients for their confidence. The authors wish to thank Dr. T.L.S. Visscher for his useful comments.
Download Provisional PDF Here
Article Type: Review Article
Citation: Govers E, Slof E, Verkoelen H, Ten Hoor-Aukema NM, KDOO (2015) Guideline for the Management of Insulin Resistance. Int J Endocrinol Metab Disord 1(4): doi http://dx.doi.org/10.16966/2380- 548X.115
Copyright: © 2015 Govers E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access