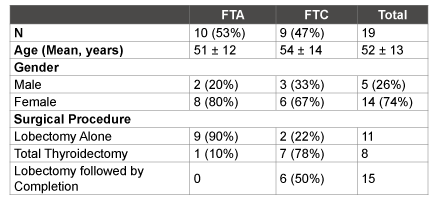
FTA, follicular thyroid adenoma; FTC, follicular thyroid carcinoma
Table 1: Patient Demographic Data comparing FNA samples of FTA and FTC lesions

Claire E Peeples1 Robert Simon1 Sapna Nagar1* Hugh S Chung1 Samreen Ahmed2 Bryan Thibodeau2 George Wilson2 Graham Long1 Peter Czako1
1Department of Surgery, Beaumont Health System, Royal Oak, MI, USA*Corresponding author: Sapna Nagar, MD, Department of Surgery, Beaumont Health System, 3601 West Thirteen Mile Road Royal Oak, MI,USA, E-mail: sapna.nagar@beaumont.edu
Objective: Fine needle aspiration (FNA) is a procedure used in the diagnosis of thyroid nodules. A definitive diagnosis is not possible when FNA shows follicular cells, and therefore a surgical intervention is necessary. Identifying genetic expression patterns in FNA samples of indeterminate thyroid nodules could assist in distinguishing benign from malignant follicular thyroid lesions.
Methods: Patients with follicular cells on FNA and a pathologic diagnosis of either follicular thyroid adenoma (FTA) or carcinoma (FTC) were included. Thyroid tissue was collected at the time of definitive surgery. Quantitative reverse transcription polymerase chain reaction (qRT-PCR) was used with an array profiler including 84 genes involved in transformation and tumorigenesis. RT2 Profiler PCR array data analysis software identified fold-change based upon ΔΔCt calculations. Gene expression was normalized to five housekeeping genes.
Results: Nineteen patients were included: 10 with FTA and 9 with FTC on post-operative pathology. In the FTC group, 11 genes had greater than 2-fold up or down-regulation relative to the adenoma group; and two genes reached statistical significance, caspase-8 and IL-8 (p ≤ 0.05). Utilizing the Sub-Network Enrichment Analysis (SNEA) algorithm, sub-networks of genes involving the transforming growth factor (TGF) family and peroxisome proliferator-activated receptor delta (PPARδ) family were both highly regulated.
Conclusions: Our preliminary data identify two potential genes that may aid in differentiating FTC from FTA, and demonstrates a potential role for qRT-PCR of FNA samples. This may contribute to the workup of thyroid nodules to ultimately guide the treatment of indeterminate follicular lesions.
Fine needle aspiration; Follicular thyroid adenoma; Follicular thyroid carcinoma; Cancer gene profiling; Thyroid nodules
Abbreviations: FNA: Fine Needle Aspiration; FTC: Follicular Thyroid Carcinoma; FV-PTC: Follicular Variant of Papillary Thyroid Carcinoma; FTA: Follicular Thyroid Adenoma; GEC: Gene Expression Classifier; RNA: Ribonucleic Acid; cDNA: Complimentary Deoxyribonucleic Acid; qRTPCR: Quantitative Reverse Transcription Polymerase Chain Reaction; Ct: Cycle Threshold; B2M: Beta-2 Microglobulin; HPRT1: Hypoxanthine Phosphoribosyl transferase 1; RPL13A: Ribosomal Protein L13A; GAPDH: Glyceraldehyde 3-Phosphate Dehydrogenase; ACTB: Actin, Beta is a protein coding gene; PCR: Polymerase Chain Reaction; SNEA: Sub-Network Enrichment Analysis; CASP8: Caspase 8; IL8: Interleukin 8; TGF: Transforming Growth Factor; PPARδ: Peroxisome Proliferator-Activated Receptor Delta; TRAIL: Tumor Necrosis Factor-Related ApoptosisInducing Ligand; TNF- α: Tumor Necrosis Factor-Alpha; TNFR: Tumor Necrosis Factor Receptor; CD40: Cluster of Differentiation 40 is a protein found on the surface of antigen presenting cells required for their activation; NF- κB: Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells; TGF- β: Transforming Growth Factor Beta
Approximately 5-10% of the general population is diagnosed with a thyroid nodule in their lifetime. Most thyroid nodules are benign; however, ruling out malignancy is necessary as this affects patient treatment and outcomes [1]. Fine needle aspiration (FNA) remains an integral component in the evaluation of a thyroid nodule, and has reduced the rate of thyroid surgery for patients with benign nodules. Previous experience has demonstrated that up to 86% of resected nodules were benign [2]. The benefits of FNA-directed evaluation of a nodule include simplicity of the procedure, economic efficiency and overall reliability to distinguish between benign and malignant disease [2-4]. Based on FNA results, a clinician makes informed decisions regarding further management options, including observation, repeat FNA or surgical intervention. While FNA is simple and accurate, there are particular scenarios where differentiation of benign and malignant cytology becomes difficult.
Clear recommendations can be made for FNA results that are Bethesda categories II, V or VI. Category III and IV lesions comprise up to 30% of all FNA biopsies, and may or may not harbor malignancy [5]. General guidelines advocate for surgical resection for these lesions to determine capsular or vascular invasion, which is consistent with malignancy. Of the lesions resected, only 15 to 30% are malignant follicular thyroid carcinoma (FTC) or follicular variant of papillary thyroid carcinoma (FV-PTC) [2,6]. The majority of pathologic findings are either follicular thyroid adenoma (FTA) or hyperplastic proliferations of follicular cells in a multi-nodular goiter [2]. Because most of these indeterminate nodules are benign on post-operative pathology, a surgical procedure could be avoided if more precise pre-operative testing were available.
A novel FNA-based assay in the form of a gene expression classifier (GEC) was developed by Afirma (San Francisco, CA) [7]. This was validated with encouraging results in analytical consistency and clinical applicability as a rule out test due to high sensitivity [5,8]. Another FNAbased assay that has yet to be independently validated is miRInform by Asuragen (Austin, Tx). This has been studied and has a high specificity, where one can rule out malignancy in indeterminate thyroid lesions [9]. All these tests show promise to help classify indeterminate lesions, but have yet to gain widespread clinical use and may benefit from additional genetic markers.
Examining follicular thyroid lesions and their FNA samples for differential expression of particular genes implicated in oncogenesis may lead to identification of more biomarkers that can discriminate malignant follicular lesions from benign lesions. This study aims to identify candidate genetic markers using a wide array of previously identified genes implicated in carcinogenesis that may potentially distinguish between FTA and FTC, thereby leading to their potential use in a biomarker panel that may be used in the pre-operative clinical setting of indeterminate thyroid nodules.
The study was approved by our institutional review board, and consent was obtained from all patients prior to testing. From October 2009 to June 2011, all patients with thyroid nodules demonstrating follicular cells on pre-operative FNA were included. Patients were recommended surgical intervention by a single surgeon in the form of a thyroid lobectomy, total thyroidectomy, or lobectomy followed by completion thyroidectomy, based on current guidelines and a discussion between the patient and surgeon. Immediately following surgical resection, an ex vivo FNA was obtained of the nodule and the tissue was immersed in ribonucleic acid (RNA) later at room temperature for 24 hours and then stored at -80°C. Histopathologic diagnosis of all surgical specimens was confirmed by an independent, board-certified pathologist. If there was variability between the pre-operative FNA and the ex vivo FNA they were not included in the study analysis. Post-operative surgical specimens were analyzed and designated as either follicular or Hürthle cell adenoma or follicular or Hürthle cell carcinoma.
Tumor tissues were prepared for RNA extraction by Polytron homogenization of 3 mg of tissue. Cellular material from tumor tissue was subjected to RNA extraction using an RNeasy Micro Kit (Qiagen, Valencia, CA) and complimentary deoxyribonucleic acid (cDNA) was synthesized by reverse transcription using 50 ng of RNA with the RT2 Preamp cDNA synthesis Kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol.
Samples were subjected to quantitative reverse transcription polymerase chain reaction (qRT-PCR) using the RT² Profiler PCR Cancer Pathway Finder Array (SABiosciences, Valencia, CA). This array profiles the expression of 84 genes involved in transformation and tumorigenesis. Gene expression levels were quantified using the Realplex Mastercycler system (Eppendorf, Hauppauge, NY). The following thermo-cycling condition was used: 95°C for 10 min, 40 amplification cycles of 95°C for 15 seconds/60°C for 1 min, followed by a melt curve. Data was analyzed using the ΔΔCt method (ΔΔCT = 2-[(Ct malignant sample-Ct malignant housekeeping gene) – (Ct adenoma sample-Ct adenoma housekeeping gene) fold-change calculations. Gene expression was normalized to the average expression of 5 housekeeping genes (B2M, HPRT1, RPL13A, GAPDH, ACTB). Polymerase chain reaction (PCR) array results were imported into Ariadne Pathway Studio for sub-network enrichment analysis (SNEA). SNEA utilizes all data points to discover highly regulated gene expression sub-networks.
Due to the rare incidence of FTC, there were only nine FTC samples that we encountered at our institution during the study time period. During that same time period, the first ten FTA samples were collected and used for analysis. All of the normalized values and statistical analyses to generate p-values from t-tests were determined using Data Assist software v 3.01 (Life Technologies, Carlsbad, CA).
A total of 19 patients were studied: 10 with FTA and 9 had FTC on postoperative pathology. Females constituted the majority of patients (74%), and mean age of the entire cohort was 52.4 ± 13 years. History of previous cancer, family history of thyroid disease, and history of hypothyroidism or hyperthyroidism were similar between groups. Patients with a suspected diagnosis of carcinoma on FNA were more likely to undergo a total thyroidectomy or lobectomy with completion thyroidectomy (p<0.0001). Patient demographics are listed in Table 1.
Genetic expression was obtained using the RT2 Profiler PCR Cancer Pathway Finder Array of 84 specific genes shown on Table 2. Percentages of genes that demonstrated expression in each sample were sub-divided into four groups by cycle threshold values (Ct): high expression (Ct<25), intermediate expression (Ct 25-30), low expression (Ct 30-35), and no detectable expression. The Ct value is inversely proportional to the gene expression, in other words, if a gene is highly expressed it will take fewer cycles to reach the set threshold compared to a gene that has low expression. Among FTA samples, 17% had high expression, 42% had intermediate expression, 29% had low expression, and 12% had no detectable expression. The FTC samples demonstrated a similar distribution, where 24% demonstrated high expression, 40% had intermediate expression, 24% had low expression and 12% no expression (Figure 1).
There were 11 genes in the FTC group that had at least a 2-fold differential expression (up- or downregulation) relative to the FTA samples (Table 3). Nine of these were downregulated; however, only caspase 8 (CASP8) and interleukin 8 (IL8) demonstrated significant downregulation of genetic expression in the FTC group (p ≤ 0.02) (Figure 2).
Utilizing the results of all 84 genes and SNEA, sub-networks of genes where expression is controlled by members of the transforming growth factor (TGF) family and by peroxisome proliferator-activated receptor delta (PPARδ) were found to be highly regulated (Figure 3). CASP8 is downregulated by the TGF family, while IL8 is downregulated by the PPARδ family
The results of this study demonstrate that it is possible to effectively analyze the limited tissue obtained from an FNA of a follicular lesion. The current study analyzes indeterminate follicular lesions, utilizing an FNA-based assay and qRT-PCR analysis, and identified two potential gene candidates which may assist in distinguishing FTC from FTA: IL8 and CASP8. Overall, both genes demonstrated significantly decreased levels of expression in the FNA samples of follicular thyroid carcinoma compared to the FNA samples from follicular thyroid adenoma.

FTA, follicular thyroid adenoma; FTC, follicular thyroid carcinoma
Table 1: Patient Demographic Data comparing FNA samples of FTA and FTC lesions
Table 2: Genes and their functions included on RT2 Profiler PCR Cancer Pathway Finder Array
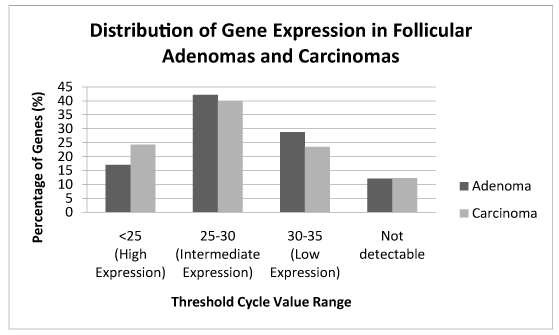
Figure 1: The percentage of genes that demonstrated expressions are shown and the Ct ranges for follicular adenomas are compared to Ct ranges for follicular carcinomas. The distribution is similar between FTA and FTC.
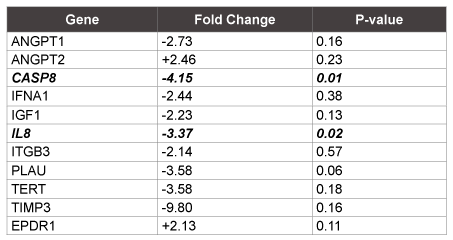
Table 3: Reference genes with at least a 2 fold change and their respective P-values. Only Caspase 8 and IL-8 reached statistical significance.
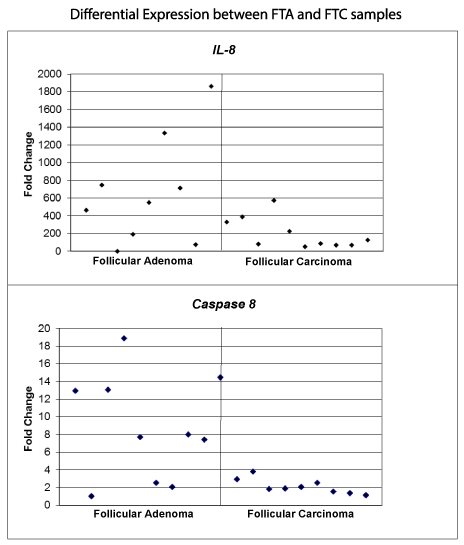
Figure 2: Fold change differential expression of IL-8 and caspase 8 in FTA vs. FTC samples. The expression values are presented as relative fold difference to the lowest expression level of IL-8 and caspase 8, respectively, and normalized to the average of reference genes. IL-8 had an average 3.37 fold decrease for follicular carcinoma compared to follicular adenoma and caspase 8 had an average 4.15 fold decrease for follicular carcinoma compared to follicular adenoma.
The importance of CASP8 and IL8 in thyroid disease is possibly due to their involvement in angiogenesis, apoptosis, inflammation and cell senescence, which could explain their role in helping to distinguish malignant from benign lesions. In a mechanism initiated by tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), procaspase-8 is cleaved into its active form, caspase-8, which then promotes apoptosis [10]. It has been observed that this TRAIL-initiated cascade particularly targets cancer cells [11]. Lee et al. [12] demonstrated TRAIL-induced CASP8 mediated cytotoxicity in malignant fibrous histiocytoma. Similar to these findings, a reduction of caspase8 potentially abates the level of cell destruction and could lead to tumorigenesis, a finding consistent with the expression observed in the carcinoma samples.
Similarly, IL8 has been implicated in carcinogenesis due to the relationship with chronic inflammation and subsequent transformation to tumor cells in pancreatic and colon cancer [13,14]. However, it is not clear why IL8 would have decreased expression in the carcinoma samples, considering its pro-inflammatory effect. A possible explanation for the reduced IL8 levels in this study is based on the established homology between tumor necrosis factor-alpha (TNF-α) and TRAIL [15]. In cervical cancer cells, TNF-α and tumor necrosis factor receptor (TNFR)- associated factor upregulate IL8 levels through CD40 and the NF-κB pathway, and TRAIL-induced expression of IL8 has been demonstrated in human intestinal epithelial cell lines [16,17]. If TRAIL expression was decreased, IL8 may also be decreased as a result.
The interrelated pathways between TRAIL and TNF-α may support our basis of TRAIL as a central component in these observations. A decrease in TRAIL levels could provide reason for both the IL8 and CASP8 reductions. TRAIL is known to activate cell death in malignant cells while sparing normal cells and has also been shown to initiate apoptosis in premalignant cells [10]. While inhibiting the apoptosis mechanism promotes tumorigenesis, one may expect an inflammatory factor such as IL8 to have increased expression. However, a down-regulation of IL8 could be due to a disturbance in an upstream component. TRAIL deficiencies have been implicated in disruption of autoimmune and cell cycle regulation [18,19]. Therefore, it is possible that an inherent decrease in TRAIL expression ultimately leads to lowered CASP8 and IL8 levels, which is what we observed in our FTC samples. Ultimately, definitive conclusions regarding the TRAIL pathway requires direct measurements of the genetic marker within the follicular thyroid cancer specimens, and this may be an area for future research.
To help with interpretation of genetic expression levels and disease states, gene set enrichment methods have been introduced [20]. SNEA was developed to identify gene sets with significant concordant changes in expression between two conditions, i.e. adenoma versus carcinoma. Each sub-network consists of a central entity that regulates the expression of downstream genes. Therefore, if the downstream expression targets contain more differentially expressed genes than expected by chance, the central entity is likely one of the activated regulators of the differential expression profile [20]. In our SNEA analysis, we were able to demonstrate two gene sub-networks that were highly regulated: expression targets of the TGF gene family and expression targets of PPARδ. PPARs are nuclear receptors that function as transcription factors, and PPARδ exhibits anti-inflammatory and anti-carcinogenic effects through the β-catenin pathway. Transforming growth factor-beta (TGF-β) has been found to inhibit both the function and proliferation of epithelial cells; in tumor cells, these functions are reduced due to changes in the signaling pathways. This leads to uncontrolled proliferation and stimulation of invasion, metastasis and angiogenesis. The role of TGF-β has been studied in thyroid carcinoma; however, studies remain inconclusive [21]. While TGF and PPARδ may not have been shown thus far to have a direct relationship to the development of follicular carcinoma, perhaps a mechanism in these gene families is associated due to the upregulation of both IL8 and CASP8. These results point to a potential field for future investigation.
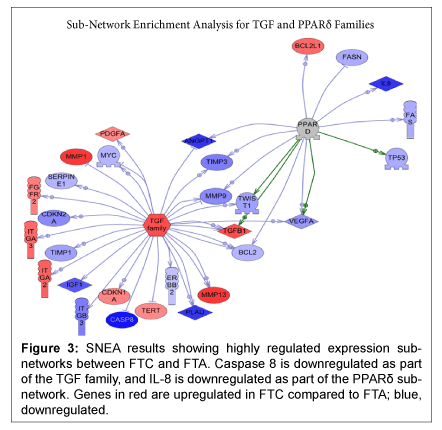
Figure 3: SNEA results showing highly regulated expression subnetworks between FTC and FTA. Caspase 8 is downregulated as part of the TGF family, and IL-8 is downregulated as part of the PPARδ subnetwork. Genes in red are upregulated in FTC compared to FTA; blue, downregulated.
The limitation of this study is the small sample size. There were several other biomarkers approaching differences of statistical significance. Should a greater power be achieved, the interrelated pathways of the biomarkers could further support our results.
Our preliminary data demonstrate the potential for genetic discrimination between FTC and FTA using qRT-PCR in FNA samples. We highlight two possible candidate markers in CASP8 and IL8. In addition, gene expression sub-networks centered on TGF and PPARδ are highly regulated in FTC, which shows promise for future research. This assay may prove valuable as a diagnostic tool to aid in the diagnosis and treatment of patients with indeterminate follicular lesions in the preoperative setting.
Download Provisional PDF Here
Article Type: Review article
Citation: Peeples CE, Simon R, Nagar S, Chung HS, Ahmed S, et al. (2015) Using Cancer Gene Profiling to Distinguish Benign from Malignant Follicular Thyroid Lesions. Int J Endocrinol Metab Disord 1(2): doi http:// dx.doi.org/10.16966/2380-548X.105
Copyright: © 2015 Peeples CE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Publication history:
All Sci Forschen Journals are Open Access