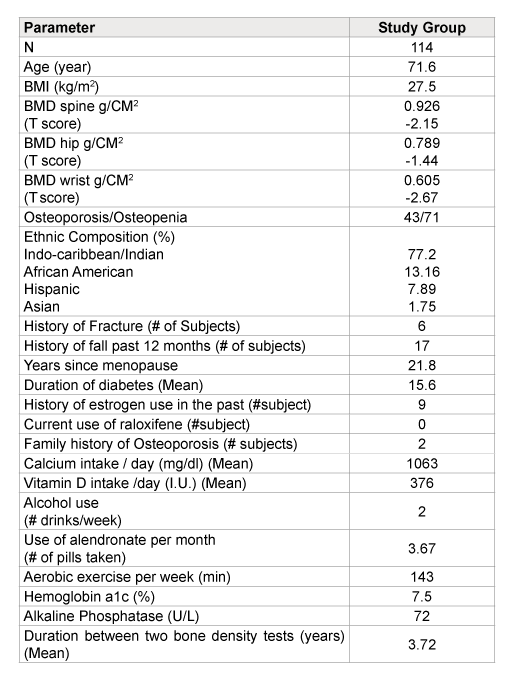
Table 1: Baseline characteristics comparing the study patients

Effect of Alendronate on Bone Mineral Density in Post Menopausal Women with Type 2 Diabetes Mellitus
Issac Sachmechi* Saman Ahmed Jalaja Joseph David Reich Lucien Cardinal Paul Kim
Division of Endocrinology, Department of Medicine, Mount Sinai Services, Queens Hospital Center, USA*Corresponding author: Issac Sachmechi, Queens Hospital Center/ Icahn School of Medicine at Sinai, 82-68 164th Street, Department of Medicine, Jamaica, NY 11432, USA, Tel: 718-883-4061; Fax: 718-883-6124; E-mail: Sachmeci@nychhc.org
Aim: To perform a retrospective study to determine the effect of Bisphophanates on bone mineral density (BMD) in the late post-menopausal (PM) osteoporatic and osteopenic women with Type 2 Diabetes Mellitus (DM).
Method: In this retrospective case control study, 114 PM diabetic women over age 65 who took alendronate for at least 3 years, 43 of them had osteoporosis and 71 with osteopenia. The efficacy of therapy was measured by comparing a minimum of two BMD studies.
Result: The study (n=114) demonstrated statistical significant BMD gain (+3.1% and +1.9%) at the spine and hip respectively but statistically significant loss of BMD (-9.8%) at forearm (FA). There was statically significant correlation between body mass index (BMI) and BMD, in spine and hip but not in FA. We found negative correlation between A1C and BMD at all 3 sites, with a statically significant correlation observed at FA.
Conclusion: It appears that Bisphosphonates are not effective in preventing bone loss in the FA of diabetic post-menopausal women. In addition bisphosphonates therapy resulted in significant gains of BMD at the spine and hip. A larger study should be done to compare bisphosphonates to other modalities of therapy for osteoporosis in diabetic post menopausal patients.
Post menopausal women; Diabetes; Osteoporosis; Alendronate; Bone mineral density
Abbreviations: BMD: Bone Mineral Density; PM: Post Menopausal; T2DM: Type 2 Diabetes Mellitus; FA: Forearm; FIT: Fractures Intervention Trial; DXA: Dual-energy x-ray Absorptiometry; TZD: Thiazolidinedione; NTX: N-terminal Collagen Cross Linked Peptide; CTX: c-terminal Collagen Cross Linked Peptide
Patients with type 2 diabetes mellitus (T2DM) have a normal or higher bone mineral density (BMD) compared with their age matched peers [1]. Several factors likely contribute to this observation. Insulin is anabolic to bone, and the hyperinsulinemic state associated with T2DM may also promote increased bone mass [2]. BMD is strongly associated with body weight, and patients with T2DM tend to be overweight or obese. Low BMD is a known risk factor for the development of osteoporosis and fracture [3].
Diabetes has been associated with an increased risk of fractures [4]. This increased risk is not necessarily mirrored by changes seen in bone mineral density (BMD), in accordance with the normal or higher than average BMD reported in patients with T2DM [4].
Data regarding the efficacy of current osteoporosis treatments in patients with diabetes are limited. Data from the MORE trial has shown that raloxifene is effective in preventing vertebral fractures in older women with diabetes mellitus [5].
Four long term studies demonstrate mixed results in changes in BMD on alendronate therapy among postmenopausal women with diabetes [6-9]. Two studies, one by Dagdelen et al. [6] and the other the Fracture Intervention Trial (FIT) [7], showed different results in terms of the effects of alendronate on BMD in this population. For example, the FIT study demonstrated BMD improvement among DM subjects (n=136) at the spine and hips, while the Dagdelen study showed BMD loss among DM subjects (n=26) at the hip and forearm compared to the control groups without DM.
A smaller study by Iwamoto et al. [8] examined the effects of alendronate on postmenopausal osteoporotic Japanese women with (n=16) and without T2DM (n=135). The results showed both groups of patients had statistically significant gains in BMD at the lumbar spine after 3 years. There was no placebo arm, and BMD at non-vertebral sites was not assessed.
Finally, in a study by Ikeda [9] 24 postmenopausal diabetic patients were followed for 5 years. The study group treated with alendronate (n=12) demonstrated no significant BMD loss at the forearm (FA), while the control group (n=12) demonstrated significant forearm BMD loss after 5 years.
The objective of this retrospective study was to determine the effects of bisphosphonates on BMD in late postmenopausal osteoporotic and osteopenic women with T2DM.
The data was collected from a public municipal hospital outpatient clinic via electronic medical records. The study groups (n=114) was selected using inclusion and exclusion criteria, as described below.
As per exclusion criteria, 58 patients from the study group were excluded.
Data collection was started in those patients who just started Bisphosphonate after having the initial BMD. We will also look at the correlation between each one of these parameters at baseline: (1) BMI, (2) A1C, (3) age, and BMD. Also look at the correlation delta A1C overtime against change of the BMD. We will perform sub analysis of diabetic patients who were on pioglitazone to see if its use will affect the response to bisphosphonate therapy in subjects with diabetes.
BMDs of the lumbar spine, femoral neck, and right forearm were measured by dual-energy X-ray absorptiometry (DXA) using a Lunar densitometer. The follow up DXA was done within two to four years. The femoral neck BMD value was a divided average of the left and right hip BMD
The participants who met the inclusion criteria received a phone interview, after obtaining verbal consent, on their medical history, risk factors for osteoporosis, race, medications including the use of thiazides, calcium and vitamin D supplements, physical activity, and history of fractures we recorded the age, BMI, of each patient at the beginning of the study and the average A1C during period of the study.
The statistical analysis was done using Minitab version 14. Pair T test was used for the main data analysis and then two sample T test was used for sub-group analysis. Pearson correlation was performed to analyze correlation between variables.
Table 1 shows baseline characteristics of the study patients, and demonstrates their ethnic group composition.
The summary of this study is shown in Table 2, which demonstrated non-statistical significant BMD gain (+3.8% and 2.6%) at the spine and hip but statistically significant loss of BMD (-10%) at forearm (FA), in the osteoporotic group. BMD gain of (+2.75% and +1.6%) in spine and hip respectively, and loss of BMD (-9.6%) in FA of the osteopenic group.
Table 3 demonstrates the combined group of osteoporosis and osteopenia patients with DM (combined study group n=114). The combined groups also show statistically significant BMD losses (-9.8%) at the forearm but non-statistically significant BMD gains at the spine (+3.1%) and a non-statistically significant BMD gain (+1.9%) at the hip. Figure 1 shows the data from Table 2 graphically.

Table 1: Baseline characteristics comparing the study patients
No fracture was repeated in the study patient during the study period. Looking at factors, like BMI and A1C and Age that could predict BMD loss. We found a statistically significant correlation between BMI and BMD, in spine and hip but not in the FA, and we found negative correlation between A1C and BMD at all 3 sites, especially at the FA, which was statistically significant. The results demonstrate as a1c decrease over time was correlated with BMD improvement at all three sites especially at the hip in Table 4. This result suggests improvement of glycemic control in post-menopausal diabetic women is associated with improvement of BMD.
Age was negatively correlated with BMD at the hip and FA but there was no correlation between age and BMD at the spine (Table 4).
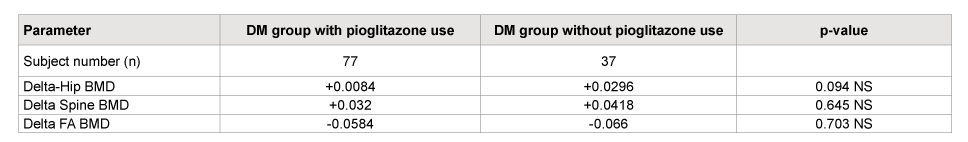
Subanalysis of study patient, who were treated with pioglitazone, did not show a statistically significant difference in BMD compare to nonpioglitazone treated group (Table 5).
In this retrospective cohort, we demonstrated a significant decrease in forearm but significant BMD gains in the spine and hip in alendronate-treated late postmenopausal osteoporotic women with T2DM (control group) over a mean period of 3.72 years. In this study we found, a trend toward a significant increase in BMD at the spine of 3.6% and the hip of 2.6% in the study group (Table 3 and Figure 1). There was a statistically significant decrease of 10.1% in BMD at the FA. It appears that bisphosphonates are not effective in preventing bone loss in FA of diabetic post menopausal woman.
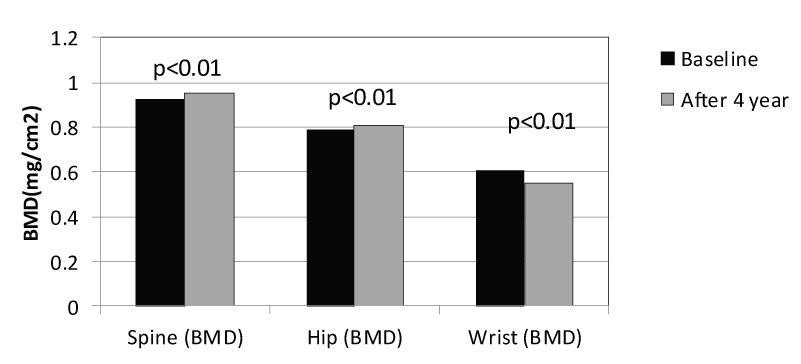
Figure 1: Change in BMD After 4 Years of Alendornate Use
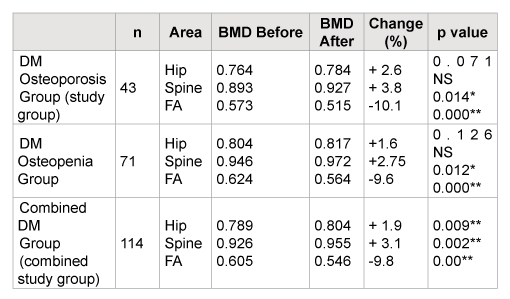
n= number of patients, (BMD in g/CM2 ), ns= non-significant, p<0.05*, p<0.01**
Table 2: BMD Pre and Post Bisphosphonate Therapy in post-menopausal diabetic woman with osteopenia, osteoporosis combined and groups
We compared our study results to the outcomes of other studies in Table 3. However, the data regarding the effects of bisphosphonate therapy on BMD among post-menopausal women with diabetes is very scarce (Table 3).
Our combined study group patients (n=114) had a statistically significant BMD gains at the spine and hip, while the FIT study patients (n=136) had statistically significant BMD gains at the spine and hip without reporting a BMD change in forearm. Therefore, our study group’s significant BMD loss at the forearm cannot be compared.
Since the measurement of BMD at the forearm is more prone to error depending on position, the BMD was performed by the same bone densitometer, by the same technician. Although not every patient had level of 25(OH) D measured but all patients were on Vit D supplement with mean supplement of 376 IU day. Therefore, this proportional loss of BMD in forearm can’t be explained by possibility of Vit D deficiency.
In the study by Dagdelen, patients (n=26) had statistically significant BMD gains at the spine but statistically significant losses at the hip and forearm compared to the control group (n=26) after 5 years use of alendronate therapy (Table 3).
In the study by Iwamoto [8], there was a reported significant increase in spine BMD, but there was no data on hip or forearm BMD. In the study by Ikeda [9], which looked only at FA BMD, there was a demonstrated non-statistically significant decrease in FA BMD in the diabetics treated with a bisphosphonate, while our study showed a significant decrease in FA BMD in patients with T2DM. Examining all of these studies, one can conclude the effect of bisphosphonate therapy on changes in BMD in post-menopausal women with diabetes is variable and difficult to compare, and that the studies, that looked at the FA; showed either non-statistically significant decrease of BMD [9], or statistically significant BMD loss [6], and in our study
We postulate a few factors might have contributed to the different results seen between our study and other studies:
First, our study population is different from the populations that were studied in the FIT trial by Keegan [7] (mainly Caucasian women) and in the Iwamoto [8] and Ikeda studies [9] (exclusively Japanese), while our study population was mainly African American, South Asian and Hispanic (Table 2). The differences in race and ethnicity may have some effect on different outcomes that we see between our study and the others.
Second, glycemic control during the study period (over 4 years in each study) might have produced different outcomes. Hyperglycemia decreases osteoblast function, bone turnover and bone density. Some of the studies do not report the level of glycemic control in their diabetic study subjects. In our study, the mean hemoglobin A1c (A1c) of the diabetic subjects was 7.5%, suggesting reasonable, but not exceptionally tight glycemic control. We found that the A1C was negatively correlated with BMD at all the 3 sites, with very significant correlation at FA. The result demonstrates that as A1C decrease over time, was correlated with BMD improvement at all three sites especially at the hip (Table 4). This result suggests improvement of glycemic control in post-menopausal diabetic women is associated with improvement of BMD
In addition, BMI differences at baseline might have contributed to different study outcomes. In the studies by Iwamoto [8] and Ikeda [9], baseline BMI and BMD were both lower than in our study.
In this study we confirmed that BMI was positively correlated with BMD in the spine and the hip but not in the FA. Age was negatively correlated with BMD at the hip and FA, but there was no correlation between age and BMD at the spine. These results explains why our diabetic post menopausal patient gain statistically significant BMD at spine but lost at FA. The FA may not be subject to the same gravitational force that spine and hips are, this is exceptional true in sedentary individual. This may explain that lack of correlation between BMI and BMD at the FA, it may also nigate any protective effect of, excess body weight on BMD at the FA, it may also explain the failure of Bisphosphonate to affect a positive increase in BMD at FA. In addition, large changes in BMD of the FA, can be influenced by changes in techniques of measuring BMD.
Our study data adds useful information to the study of diabetes and osteoporosis in terms of the effects of bisphosphonate drugs in this population. This is an area that has been relatively unstudied, and, as a result, there is very limited data available. We believe our study contains the following strengths:
First, our study had a larger number of patients compared to the studies by Dagdelen [6], Iwamoto [8] and Ikeda [9].
Second, this is the first study examining a largely non-white population, in particular, a largely South Asian population. To the best of our knowledge, this is the first study done in a largely South Asian diabetic population with osteoporosis, examining the effects of bisphosphonate use on BMD. Diabetes is a worldwide epidemic, and it is especially prevalent among South Asians.
Third, our study is only the fifth to examine how diabetes mellitus could potentially affect the action of bisphosphonates in terms of their effect on BMD in post-menopausal women with T2DM and osteoporosis.
Fourth, this is the first study in post-menopausal diabetic patient treated with bisphosphonates that looked at correlation between A1C and BMD at 3 sites: spine, hip and FA.
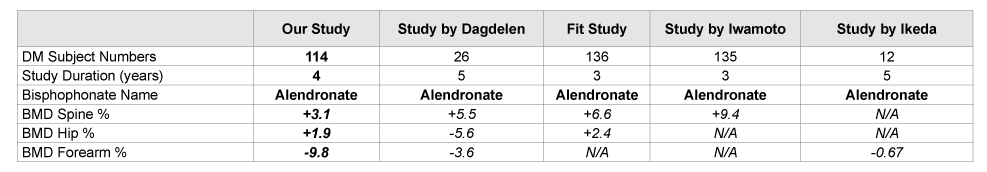
Table 3: Comparison of our study outcome to other studies’ outcome regarding BMD change among post-menopausal women with diabetes after bisphosphonate use
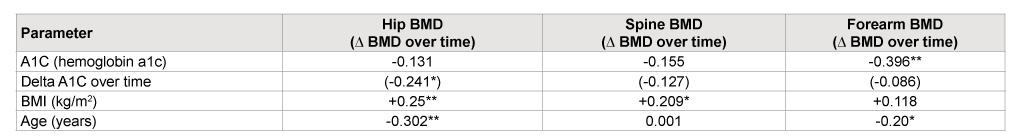
Table 4: Pearson correlation with three BMD (bone mineral density in g/CM2 ) sites and delta BMD change (∆ BMD) against the study subjects’ parameters at baseline and over time (p<0.01**, p<0.05*)
(NS = non-statistically significant)
Table 5: Sub-analysis for the study subjects’ BMD change (delta) with and without pioglitazone use comparing with two sample T test
Fifth, this study is the first study to perform a subgroup analysis of diabetic patients who were on pioglitazone to see if a Thiazolidinadione (TZD) would affect the response to bisphosphonate therapy in subjects with diabetes. The subgroup who were on pioglitazone (n=77) showed no significant BMD change compared to the subgroup not treated with pioglitazone as part of their diabetes therapy (n=37) after four years of use.
Our study results have to be viewed in light of certain limitations. This study is a retrospective analysis, and it is not large enough and the duration of follow up is not long enough to adequately assess fracture risk with a great degree of statistical power. Also, markers of bone formation and resorption were not available.
We postulate that duration of diabetes, glycemic control, BMI, advanced glycation end products (AGEs), and oxidative stress might explain different outcomes of our study compared to others.
Our diabetic subjects had a longer duration of T2DM (15 years) compared to patients in the FIT study (11.4 years) and the study by Dagdelen (7 years), and other studies did not report the duration of diabetes in their study subjects.
Duration of diabetes affects BMD of post-menopausal women with diabetes. For example, in a study by Viégas et al. [10], the duration of T2DM correlated with osteoporosis-related fractures, which increased from 12.5% in those diagnosed with diabetes mellitus within the previous 5 years to 32.8% in those who had been diagnosed for more than 10 years. This is in accordance with studies that have shown a relationship between a higher rate of fractures and a longer duration of T2DM [11].
Their study confirms positive correlation between BMI and BMD in the spine and the hip but not in FA. The baseline BMI is different in all the studies compared to this study.
AGEs accumulate in patients with diabetes, especially with longer duration, affecting bone collagen, and have been proposed as a factor contributing to bone fragility [12].
Oxidative stress may promote increased marrow adipogenesis and decreased osteoblastogenesis that enhanced by the Wnt pathway [13]. Sclerostin, a product of osteocytes, antagonizes the Wnt signaling pathway, resulting in inhibition of osteoblasts [14]. Higher sclerostin levels have been reported in type 2 diabetes [15]. Diabetes, therefore, may be associated with changes in osteocyte function and the Wnt pathway, possibly contributing to bone fragility and an increase in fracture risk [16]. Marrow fat also produces factors that may directly affect osteoblasts and osteoclasts. In co-cultures with marrow adipocytes, osteoblast activity was inhibited [17], possibly due to release of free fatty acids [18]. Fat in the marrow, like other fat depots, produces inflammatory cytokines that can promote osteoclast recruitment, resulting in bone loss [19].
It appears that bone turnover is different in cortical as compared with trabecular bone; therefore the effect of bisphosphonates on these two different bone types is not equal. It is possible that in diabetics bisphosphonate therapy increases bone density in trabecular bone, but has less effect on cortical bone, demonstrated by a loss of bone mineral density in the wrist in some studies [20].
We compared our study that was done on post-menopausal women with Type 2 diabetes to the study done by Hosking et al. which studied the effect of alendronate on BMD in post-menopausal women (n=1174) without diabetes. Loss of BMD in forearm in this was statistically significant, as in our study, after five years of alendronate use [21]; suggesting that bisphosphonate may not effect bone loss at FA, also in non-diabetic post menopausal women.
Diabetes is a disease characterized by a state of low bone turnover [22]. Antiresorptive-type drugs for osteoporosis work by decreasing osteoclast activity and thus further slowing bone turnover [23]. For example, decreases in the range of 40-50% in total and bone-specific alkaline phosphatase and urine N-terminal collagen crosslinked peptide (NTX) have been reported within 1 year after starting alendronate and risedronate [24]. Furthermore, decreases of 30-35% have been reported in bone-specific alkaline phosphatase, osteocalcin, and C-terminal collagen crosslinked peptide (CTX) with raloxifene use [25].
We can carefully hypothesize that anti resorptive drugs work less well in patients with diabetes and may perhaps lead to accumulation of old bone with microcracks and thus, result in an increase in osteoporotic fracture risk [26-30]. Some reports have suggested that atypical subtrochanteric fractures, potentially linked to bisphosphonate use, may be increased in patients with diabetes [29]. Since biphosphonates have been traditionally used for the treatment of osteoporosis, and there are contradictory results from studies of bisphosphonate use in diabetics, it is imperative that larger prospective studies in post-menopausal diabetic patients with osteoporosis should be done to clarify whether the use of anabolic medications like teraparatide or use of RANK Ligand inhibitor; denosumab (Prolia) for the use of treatment of osteoporosis are a better modalities of therapy, in this group of patients.
Our study shows that post-menopausal diabetic patients who were treated with bisphosphonates for a mean of 3.72 years showed an increase in BMD at the spine and the hip. On the other hand, our study did demonstrate very significant decreases in BMD at the FA. The study is the fifth to date on this topic, and given the myriad outcomes from some of the previous ones, one can conclude that there is a strong need to do larger, well controlled studies to confirm or dispute the results.
It appears that bisphosphonates are not effective in preventing bone loss in the FA of diabetic post-menopausal women. Large prospective studies should be done to compare the efficacy of anabolic like Forteo, Rank Ligand inhibitor: Prolia, versus antiresorptive therapies in terms of inhibiting bone mineral loss in osteoporotic post menopausal diabetic woman. As a result of this study we recommend weight bearing exercises especially for FA to minimize bone loss in post-menopausal diabetic patient.
No funding received. The author(s) acknowledge each has contributed significantly to the manuscript. The author(s) hereby confirm that neither the manuscript nor any part of it, except for abstracts less than 400 words, has been published or is being considered for publication elsewhere. There are no financial or other relationships that could lead to a conflict of interest
The authors have nothing to disclose.
Download Provisional PDF Here
Article Type: Research Article
Citation: Sachmechi I, Ahmed S, Joseph J, Reich D, Cardinal L, et al. (2015) Effect of Alendronate on Bone Mineral Density in Post Menopausal Women with Type 2 Diabetes Mellitus. Int J Endocr Metab Disord 1 (1): doi http://dx.doi.org/10.16966/2380-548X.103
Copyright: © 2015 Sachmechi I et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Publication history:
All Sci Forschen Journals are Open Access