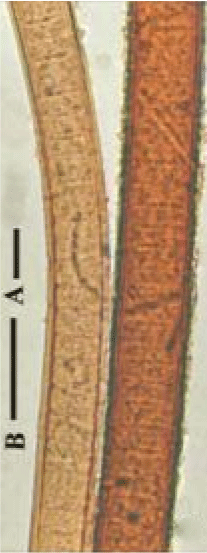
Figure 1: L. ceylanica filaments from moist rice-filed soil (after Aziz and Tanbir 1999) [14].


Abdul Aziz*
Department of Botany, University of Dhaka, Dhaka 1000, Bangladesh*Corresponding author: Abdul Aziz, Department of Botany, University of Dhaka, Bangladesh, E-mail: dr.aziz.botany@gmail.com
Filaments of Lyngbya notarisii (Menegini) Wille (Cyanobacteria), is characterized by having very thick sheath around a trichome and production of yellow-brown to reddish-brown or red pigment called scytonemin. In liquid Chu 10D medium, under a continuous light flux of 50 µE m-2s-1 (PAR) and at a temperature of about 25°C, filaments may attained about 3 cm long and upto 42 µm wide, trichomes 8-22 µm diam and sheath 6.6-14 µm diam. after 15 days. The scytonemin produced in trichomes and certain amount of which presumably absorbed by sheath layers, mostly abundant in the innermost layer as filaments grow. An old filament when placed on a glass slide with Chu 10D medium and exposed to direct sunlight for two days (11:13 hrs, day and night) at about 950 µE m-2s-1 mid-day PAR and temperature of about 35°C, reddish-brown water-soluble scytonemin oozes out through open end. The scytonemin released in the beginning formed black flakes on posterior sides after drying, while it remained in a liquid state in the anterior end indicating continuous scytonemin production, which appeared to be induced by high sun-light and temperature factors. Empty colored sheath may be obtained by increasing phosphorus in the medium inducing hormogonia release. There are as many as six possibilities of extraction of scytonemin and or making scytonemin cream depending on species reported: (a) extracting water soluble scytonemin through repeated sub-culturing of L. notarisii under direct sun-light and at a temperature above 35°C; (b) extracting pure scytonemin from trichomefree sheath by 100% acetone; (c) making the scytonemin impregnated sheath as such in to protective cream; (d) making crude scytonemin (mixed with chl. a) from whole filaments by 100% acetone; (e) making whole filament/biomass in to crude scytonemin cream by most of the scytonemin producing cyanobacteria and (f) growing scytonemin extracted and rehydrated cyanobacteria/biomass, and extracting newly synthesized scytonemin periodically from acetone tolerating cyanobacteria. Production of scytonemin protecting cream from L. notarisii would be easier and less expensive because of its water solubility under direct sun-light and temperature above 35°C.
Scytonemin; Skin Protective cream; Lyngbya notarisii; Sheath; Pigment; Growth-phases
Cyanobacteria varying from unicellular colonial to multicellular filamentous forms mostly produce yellow-brown to red thicksheath [1,2]. The color is due to the presence of scytonemin pigment to protect the cyanobacteria from near UV radiations [3]. The pigment can constitute up to 5% of cyanobacterial dry weight in cultured organisms [4]. UV-A and UV-B radiations are harmful to living systems [5,6]. Scytonemin is synthesized in cells in response to UV-A exposure, released as extracellular metabolites and accumulates within the extracellular sheath or slime of the producing organism, forming a stable, protective layer that absorbs as much as 90% of further incident radiation (λmax=370 nm in vivo, 384 nm in vitro) [7]. It is not only a photo-protectant but also reported to help in the surviving and sustaining cyanobacterial life [8] acting as an antioxidant, preventing cellular damage [9,10]. The pigment is highly stable against different stressors and performs its UV-absorbing/UV-screening activity without any further metabolic investment [11]. High culture temperature, strong light intensity and light-dark cycle (12:12 h) were found to be good for producing the oxidized scytonemin by N. commune [12]. A significant synergistic enhancement of scytonemin synthesis was observed under combined stress of heat and UV radiation [13]. Filaments of Lyngbya ceylanica from rice-filed soil differed in color, where filaments from upper part of a mat were wider and reddish-brown, while filaments from lower part were narrower and brownish-yellow but with similar thickness of sheath [14] indicating that higher light induced synthesis of scytonemin (Figure 1). The present paper describes the presence of scytonemin in relation to growth stages of L. notarisii, factors responsible for its formation and possible ways of using it in preparing protective cream.

Figure 1: L. ceylanica filaments from moist rice-filed soil (after Aziz and Tanbir 1999) [14].
Lyngbya notarisii (Meneghini) Wille 1914 (=Porphyrosiphon notarisii (Meneghini) Kutz. 1850) [1,2,15] (Cyanobacteria) growing on dry coconut palm-bark as blackish-pink layer was collected from Panchagar district, Bangladesh [14]. Mid-day light flux was about 950 µE m-2s-1 PAR measured by Li-Cor light meter model LI-185B, USA, while day temperature varied from 25 to 28°C on 25 Nov. 1995. The blackish-pink layer after soaking in water was found to contain yellowish-brown (due to scytonemin) filaments, 28 µm in diameter with 4.4 µm wide multi-layered sheath that is frayed at the tips (Figure 2).

Figure 2: Lyngbya notarisii (Meneghini) Wille 1914 (=Porphyrosiphon notarisii (Menegh) Kütz. 1850 taken from the palm bark showing details of cells and frayed sheath at the tip.
The palm-bark with L. notarisii was placed in a Petri dish containing Chu 10D medium [15] and was incubated at a continuous light flux of 50 µE m-2s-1 PAR, provided by cool white fluorescent tubes and at a temperature of about 25°C. The morphogenesis of filaments, presence of color and its distribution, thickness of sheath, etc. were studied by picking up growing and mature filaments for 15 days [15] (All figures after Aziz [15]. Bars=25 µm: Bar A is for Figures 4,6,8-10; Bar B is for figures 1,2,5,7. Figure 3 scale is in millimeter).

Figure 3: Growth of L. notarisii in liquid Chu 10D medium on a Petri dish: Pinkish-black layer on a bark produced many blue-green filaments of differing ages after about 10 days.

Figure 4: Growth stages/forms of L. notarisii in liquid Chu 10D medium on a Petri dish: A filament after about 15 days with multilayered reddish-brown sheath.
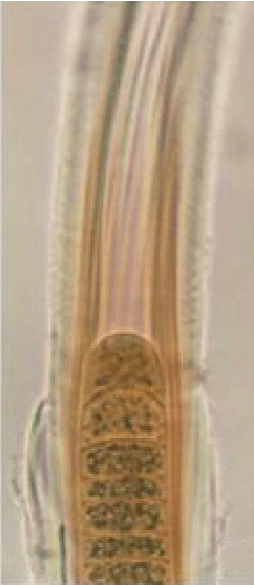
Figure 5: Growth stages/forms of L. notarisii in liquid Chu 10D medium on a Petri dish: An enlarged terminal part showing details of sheath, cell structure with cyanophycin granules and scytonemin pigment distribution.

Figure 6: Growth stages/forms of L. notarisii in liquid Chu 10D medium on a Petri dish: A filament with three trichomes or hormogonia inside the thick reddish-brown parent sheath.

Figure 7: Growth stages/forms of L. notarisii in liquid Chu 10D medium on a Petri dish: An enlarged part of the same filament with two trichomes/hormogonia showing details of all components.
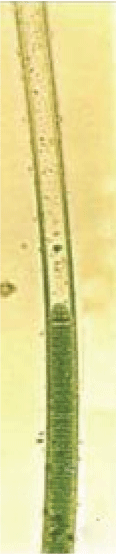
Figure 8: Growth stages/forms of L. notarisii in liquid Chu 10D medium on a Petri dish: Lyngbya-like filament from 3-4 day old culture, with narrow and uniform wide sheath.

Figure 9: Growth stages/forms of L. notarisii in liquid Chu 10D medium on a Petri dish: A false-branched Plectonema-phase after 5-6 days.
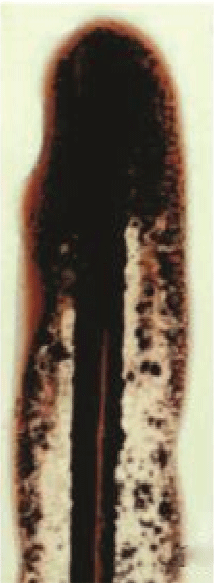
Figure 10: A 15 day old filament on a glass slide with Chu 10D after exposure to sunlight for two days: note oozing out of huge yellowbrown ‘scytonemin’ through open end, black flakes after drying along posterior part and liquid form along anterior part.
A glass slide containing a mature filament on Chu 10D medium was placed on the roof top having about 950 µE m-2s-1 PAR under full sunshine and temperature over 35°C during mid day, first week of December for studying changes of scytonemin content in the filament.
L. notarisii filaments on a bark when grown in Chu 10D medium in a Petri dish attained about 3 cm long and upto 42 µm wide with 8-22 µm wide trichomes surrounded by a sheath of 6.6-14 µm diameter having 10-12 layers after 15 days (Figures 3-7). Diameter of trichome to sheath ratio varied from 1.21:1 to 1.57:1. From many of these filaments hormogonia of 4-9 (-20) cells were released that developed into bluegreen long filaments with thin sheath having no traceable scytonemin under light microscope (Figure 8). Scytonemin presence was detected on single layered sheath at Plectonema-stage of growth (Figure 9). Under the same culture conditions, filaments become gradually thicker with multilayer sheath, and the scytonemin formed in cells is released in certain proportion and absorbed by sheath making it yellow-brown, while the rest amount remained in cells masking the blue-green trichome after 3-6 days (Figures 8,9) in to greenish-brown after 10 to 15 days (Figures 4-7).
In pictures of Lyngbya sp. CU2555, Rastogi et al. [11] showed light yellow-brown protruded trichomes from reddish-brown enclosing sheaths. The yellow-brown trichome instead of blue-green within the sheath indicated that the trichome contains scytonemin that partially masked chl. a. Similar was the case with scytonemin pigmentation in the present study for L. notarisii (Figures 4-7). Scytonemin is often found in the upper layers of microbial mats consisting primarily of Lyngbya aestuarii, in light-exposed areas and are often found in extreme environments [5]. Aziz and Tanbir [14] also observed reddish-brown filaments in the upper layer of a mat and brownish-yellow in lower ones of L. ceylanica growing on moist rice-field soils (Figure 1).
In the present study a continuous constant-light flux was used from day-light fluorescent tube-lights and scytonemin formation started approximately after 3-4 day and formation increased till 15 days incubation when hormogonia were also formed and released leaving empty sheath (Figure 5). Sometimes two to three hormogonia remained within the common sheath (Figures 6,7) indicating phosphorus sufficiency [16]. L. notarisii is non-heterocystous, so deficiency of intracellular nitrogen was likely in the batch culture under continuous light flux (high oxygen concentration). But trichome cells contain abundant cyanophycin granules, as stored nitrogen (Figures 5-7), that ruled out the possibility of nitrogen deficiency. At mature stage, though filaments show reddish-brown color, the scytonemin was not released in the medium under laboratory growth conditions of low light and moderate temperature.
A mature L. notarisii filament (like that shown in figure 4) keeping in Chu 10D medium on a glass slide was placed under full sunshine having ambient temperature over 35°C (the glass slide itself might have higher) during mid day. After two days, huge yellow-brown water soluble pigment scytonemin oozes out through open end (Figure 10). The UVR from direct sunlight and high temperature were responsible for the release of the pigment, as the pigment was not released in the laboratory. A significant synergistic enhancement of scytonemin synthesis was also observed in Scytonema sp. R77DM under combined stress of heat and UV radiation [13]. Oozing of scytonemin started from the first day and continued on the subsequent days. The scytonemin released earlier was dried forming black flakes. It appears that the trichome was continuously synthesizing and releasing UV shielding scytonemin as extracellular metabolite to protect its cells (Figure 10). The constituent of the water soluble pigment has not been analyzed where some water soluble phycobillin pigments might be present. Cockell and Knowland [17] concluded that the scytonemin formation in cyanobacteria is inherent to protect the cell and its content from photo-oxidation and lethal UV radiation. Rastogi et al. [11] observed stimulating effects of ultraviolet radiation on scytonemin production and a significant synergistic enhancement of scytonemin synthesis. Several abiotic factors, such as different wavelength bands of UV radiation, desiccation, and nutrients and salt concentration affected the production of scytonemin in cyanobacteria [18-21].
Combination of strong sun light and high temperature induced scytonemin formation and release by L. notarisii in the present study as well as by N. commune [12]. A significant synergistic enhancement of scytonemin synthesis was observed under combined stress of heat and UV radiation in Scytonema sp. R77DM [13]. Strong sun light is always associated with high temperature, so are synergistic factors. Therefore, how much the heat and light energies are individually inducing scytonemin synthesis was not ascertained. For ascertaining effect of the individual factor on scytonemin synthesis, experiments are to run at higher temperature in absence of any light and under strong sunlight at very low temperature (~0°C) with L. notarisii.
As many as six possibilities of extraction of scytonemin pigment and or making scytonemin cream may be summarized based on the present study and other reports:
Production of scytonemin protective cream from L. notarisii would be easier and less expensive because of its water solubility under direct sun-light and temperature above 35°C. The compound is highly stable against different stressors and performs UV screening activities without any further metabolic investment [11].
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Aziz A (2018) “Scytonemin” Pigment in Lyngbya notarisii (Meneghini) Wille and Possibility of Using it in Preparing Skin Protecting Cream. J Drug Res Dev 4(2): dx.doi.org/10.16966/2470-1009.145
Copyright: © 2018 Aziz A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access