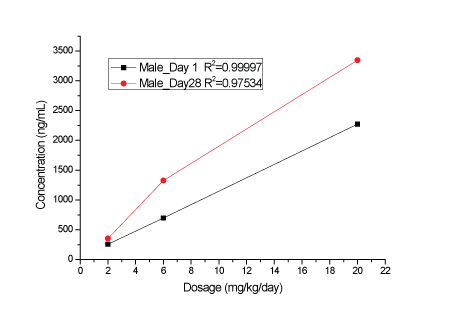
Figure 1: Cmax values of KBP-5074 in male rats on Days 1 and 28.


C Paul Chow1* JR Liu2 XJ Tan2 Fred Yang1 ZH Huang2
1KBP BioSciences USA Inc., 116 Village Boulevard, Suite 210, Princeton, New Jersey 08540, USA*Corresponding author: Paul Chow, PhD, DABT, KBP BioSciences USA Inc., 116 Village Boulevard, Suite 210, Princeton, New Jersey 08540, USA, E-mail: cpaul.chow@kbpbiosciences.com
Objective: Mineralocorticoid receptor antagonists have been demonstrated to reduce the mortality in patients with heart failure and to decrease proteinuria in patients with cardiorenal diseases. The primary objective of this study was to evaluate the preclinical safety profile of KBP-5074 to support the initiation of clinical trials in human.
Results: Genotoxicity studies of KBP-5074 included in vitro mutagenicity and chromosomal aberration studies as well as in vivo micronucleus test in CD-1 mice. KBP-5074 demonstrated neither mutagenic nor clastogenic potential. Acute oral administration of KBP-5074 to SD rats and Beagle dogs showed the maximum tolerated dose was ≥ 600 mg/kg in both species. A 4-week oral rat toxicity study was performed using 0, 2, 6 and 20 mg/kg/day. No significant clinical or behavioral signs of toxicity were observed after oral administration of KBP-5074 in any rats during the study. Treatment-related changes in clinical chemistry on Day 29 included increased hepatic enzymes, blood urea nitrogen, alkaline phosphatase and plasma potassium concentrations in the female rats. No changes in any clinical chemistry parameters on Day 29 were observed in male rats. Plasma concentrations in female rats were about 2-3 times higher than male rats. A 4-week oral dog toxicity study was performed using 0, 2, 6 and 20 mg/ kg/day. No overt clinical or behavioral signs of toxicity were observed after oral administration of KBP-5074 to male and female dogs at doses up to 20 mg/kg/day for 4 weeks. Safety pharmacology studies, including in vitro hERG assay (IC50: 17 µM) and the in vivo core battery of CNS safety pharmacology study in rats and cardiovascular safety pharmacology study in dogs conclusively demonstrated that KBP-5074 was well-tolerated at doses up to 10 and 15 mg/kg, respectively.
Conclusion: Based on the results of the above studies, the preclinical safety profile of KBP-5074 would support the First-in-Human study.
Mineralocorticoid receptor antagonists; Cardiorenal diseases; Chronic kidney disease; Systolic Blood Pressure; Hyperkalemia
KBP-5074 is a Non-steroidal Mineralocorticoid Receptor Antagonist (MRA) which blocks the binding of aldosterone, a component of the renin-angiotensin-aldosterone system. Aldosterone binds to the Mineralocorticoid Receptors (MR) in both epithelial (e.g., kidney) and non-epithelial (e.g., heart, blood vessels and brain) tissues and increases blood pressure through induction of sodium reabsorption and possibly other mechanisms. KBP-5074 binds selectively to recombinant human MR with in vitro antagonistic activity (IC50: 2.7 nM) superior than the other MRAs [1]. MRAs have been demonstrated to reduce the risk of mortality in patients with heart failure and to decrease proteinuria in patients with chronic kidney disease (CKD). Rossignol et al. [2] recently reported that MRAs improve outcomes in patients with systolic heart failures but may worsen renal function and induce hyperkalemia. High blood pressure and hypertensive kidney damage are the leading cause of morbidity and mortality in the United States [3]. Although considerable progress has been made in the treatment of hypertension and renal failure, the development of new therapy remains critical. MR is a member of the steroid receptor family and has been demonstrated to have a major pathophysiological role in the progression of kidney diseases [4,5]. The inhibition of MR signaling considerably reduces proteinuria in patients with chronic kidney disease [4,6]. Eplerenone is a highly selective aldosterone blocker developed for the treatment of hypertension and heart failure. However, MR antagonism with eplerenone causes a dose-dependent increase in serum potassium concentration [7]. The risk of hyperkalemia may also be high if the patients with renal insufficiency, diabetes and microalbuminuria were treated with currently available MRAs [8]. As a result, a novel MRA with an improved efficacy and a low potential for adverse effects would address this unmet medical need. The primary objective of this study was to evaluate the preclinical safety profile of KBP-5074 for the initiation of the First-in-Human (FIH) study.
The non-clinical laboratory studies were conducted in compliance with the current U.S. Food and Drug Administration’s Good Laboratory Practice (GLP) Regulations, 21 Code of Federal Regulations (CFR) Part 58, and China Food and Drug Administration (CFDA) GLP regulations (September2003).
Chemical Name: 2-chloro-4-[(3S,3aR)-3-cyclopentyl-7- (4-hydroxypiperidine-1-carbonyl)-3,3a,4,5-tetrahydro-2Hpyrazolo[3,4-f]quinolin-2-yl] benzonitrile
Relative Molecular Mass: 504.02 g/mol
Appearance: Yellow or greenish-yellow crystalline powder
Two in vitro and one in vivo studies were conducted in accordance with ICH guidance on genotoxicity testing for pharmaceuticals intended for human use [9,10].
Mutagenicity test in Salmonella Typhimurium: The mutagenicity of KBP-5074 was evaluated in five tester strains, i.e. TA97a, TA98, TA100, TA102, and TA1535, at dose levels of 1.5, 5, 15, 50 and 150 µg/plate. The solvent (DMSO) control and positive controls of direct mutagen Dexon at 25 µg/plate, direct mutagen sodium azide (SA) at 6 µg/plate, or indirect mutagen 2-Aminoanthracene (2-AA) at 3 µg/ plate were also included in the tests. The dose of this study was decided according to the results of a preliminary dose-range-finding study. No toxicity of the test article was noted at all dose levels up to 5000 µg/ plate for all five tester strains. Precipitation was noted at dose levels of 150-5000 µg/plate. As per the ICH guideline S2(R1), the lowest precipitating dose of 150 µg/plate was selected as the top dose in this study and 50, 15, 5 and 1.5 µg/plate were selected as lower dose levels used in this study.
Chromosomal aberration study in Chinese hamster lung fibroblast: In the pilot cytotoxicity study, the inhibition rate was 60% at 5 µg/mL of KBP-5074 and a cytocidal effect was noted at doses of ≥ 15 µg/mL when treated for 24 hours without the presence of S9. A cytocidal effect was noted at doses of ≥ 50 µg/mL when treated for 4 hours with or without the presence of S9; the inhibition rates were 39% and 36%, respectively, at 15 µg/mL. The final concentrations of KBP-5074 in this test were 20, 6 and 2 µg/mL for 4 h of treatment with the absence or presence of S9 and 5, 1.5 and 0.5 µg/mL for 24 h of treatment with the absence of S9, respectively. The vehicle control (1% DMSO) and positive controls of direct clastogen Mitomycin C (MMC) at 0.1 µg/mL and indirect clastogen Cyclophosphamide Monohydrate (CP) at 10 µg/mL were also included in the test. The cells were cultured either for 24 hours following the treatment with test or control article in the absence of S9, or for 4 hours following the treatment with test or control articles in the absence or presence of S9 and for another 20 hours after switching to normal culture medium without test or control article. Duplicate flasks for each treatment were used in this study. Cell cultures were treated with colchicine (0.01 mg/mL, 0.15 mL) for 4 hours prior to harvesting. At the end of incubation, cell cultures of each flask were harvested, treated with hypotonic solution (0.075 M potassium chloride), fixed and processed to chromosome slides. A total of 200 or 100 well-spread metaphase cells were evaluated per treatment and the chromosomal aberrations for each treatment were calculated.
Oral micronucleus test in CD-1 Mice: A total of 70 CD-1 mice were assigned to 5 groups with 10/sex in the negative control group and test article high-dose group and 5/sex in the positive control (Cyclophosphamide) group as well as the test article low- and middose groups. Bone marrow was collected at 24 h post-dose from 5/sex/ group and at 48 h post-dose from the remaining 5/sex in the negative control and high-dose groups. The high dose in this study was then set at 600 mg/kg based on the dose volume limit of 20 ml/kg and maximal solubility of KBP-5074. The doses of KBP-5074 solid dispersion in this mouse study were60, 200 and 600 mg/kg.
Rodent and non-rodent studies were conducted in accordance with Institutional Animal Care and Use Committee guidelines.
Acute and 4-week rat toxicity study: In the acute rat study, a total of 80 male and female SD rats were assigned to 4 groups (10/sex/group). The high dose was 600 mg/kg because it was the highest feasible concentration of KBP-5074 formulation. Each rat was administered a single dose of KBP-5074 at 0 (Sterile water for injection), 60, 200 or 600 mg/kg via oral gavage on Day 1. All animals were euthanized on Day 15 and examined for gross pathology.
In the 4-week study, a total of 196 SD rats (98/sex) were assigned to 8 groups based on body weight in both sexes, of which 120 rats were assigned to Groups 1, 2, 3 and 4 (15/sex/group) for the main study and 10 rats to Group 5 (5/sex) or 66 rats to Groups 6, 7 and 8 (11/sex/ group) for the toxicokinetic study. Rats were treated via oral gavage with vehicle control (sterile water for injection) in Groups 1 and 5 or KBP-5074 at doses of 2 mg/kg/day in Groups 2 and 6, 6 mg/kg/day in Groups 3 and 7, or 20 mg/kg/day in Groups 4 and 8, respectively. The first 10 rats/sex/group in Groups 1 to 4 and all animals in Groups 5 to 8 were euthanized on Day 29 and the remaining animals in Groups 1 to 4 were euthanized on Day 57. For toxicokinetic study, blood samples were collected on Days 1 and 28 from each animal at 0 (pre-dose), 0.5, 1, 2, 4, 8 and 24 hours after dosing. Blood samples were then centrifuged and plasma concentrations of KBP-5074 were analyzed using a validated LC-MS/MS methodology.
Acute and 4-week dog toxicity study: In the acute oral dog study, a total of 8 male and female Beagle dogs were assigned to 4 groups (1/sex/group). Each dog received a single oral dose of KBP-5074 at 0 (Vehicle), 60, 200 and 600 mg/kg on Day 1. All animals were euthanized on Day 15 and examined for gross pathology.
In the 4-week study, a total of 48 male and female Beagle dogs were assigned to 4 groups (6/sex/group) and received sterile water (for injection) in Group 1 or KBP-5074 Solid Dispersion at doses of 2, 6 or 20 mg/kg/day in Groups 2, 3 or 4, respectively. The first 4 dogs/ sex/group were euthanized after 4-week of dosing on Day 29 and the remaining 2 dogs/sex/group were euthanized on Day 57 following a 4-week recovery period after dosing. All animals were subjected to a complete necropsy examination and histopathological evaluation. For toxicokinetic study, blood samples were collected at 0 (pre-dose), 0.5, 1, 2, 4, 6. 8, 12 and 24 hours after dosing and analyzed by LC-MS/MS method.
Experimental designs for the safety pharmacology core battery was conducted in compliance with GLP according to guidance developed by the ICH Technical Requirements for Registration of Pharmaceuticals for Human Use (November 8, 2000) to investigate the effects of KBP-5074 on vital functions.
Effect on central nervous system function in SD rats via oral gavage: The acute CNS effects of a single oral gavage dose of KBP5074 solid dispersion on the central nervous system function in SD rats was evaluated utilizing a functional observational battery (FOB) test in SD rats. The study consisted of 40 rats (20 females and 20 males) assigned to either the control (sterile water) or one of three treatment groups (KBP-5074 solid dispersion: 1, 3 and 10 mg/kg) with 10 rats/ group (5 females and 5 males). The evaluation was performed in all treated rats within 4 h, 24 h and 48 h following administration of KBP-5074 solid dispersion compared to control rats. No treatmentrelated abnormality in rats was found in all home-cage observations, hand-held observations, open-field observations, or stimulus activity responses. The times of rearing, number of defecations, forelimb grip strength and body temperature were compared between each treated group and the control group. No abnormality was noted at 4, 24 and 48 hours after dosing.
Effects on cardiovascular and respiratory function in conscious beagle dogs: Beagle dogs (4/sex) were used to evaluate the acute effects of a single oral gavage dose of KBP-5074 solid dispersion on cardiovascular and respiratory system function via telemetry. Conscious dogs were administered either vehicle (sterile water for injection) or KBP-5074 solid dispersion (0.6, 3, or 15 mg/kg) in a Latinsquare design with a 2-day washout period. The following evaluations were conducted: mean arterial blood pressure (MBP), diastolic and systolic blood pressure (DBP and SBP), electrocardiographic parameters [PR interval, QRS interval, QT interval (QTcF interval), QRS voltage, heart rate (HR), ST voltage, Tp-e interval and RR interval], respiration frequency, tidal volume, and body temperature were recorded and analyzed at approximately 1 h pre-dosing, and 0.5 h (± 5 min), 1 h (± 10 min), 2 h (± 10 min), 3 h (± 15 min), 4 h (± 15 min), 6 h (± 30 min), 8 h (± 30 min), 12 h (± 45 min) and 24 h (± 1 h) after dosing.
In vitro mutagenicity: Turbidity was noted when KBP-5074 formulation was mixed with PBS/S9 and top agar at the dose of 150 µg /plate. However, no turbidity was noted at the end of incubation at the dose of 150 µg/plate. No turbidity was noted at other dose levels. No abnormity of background lawn was observed either with or without the presence of S9, indicating no cytotoxicity of KBP-5074 at all dose levels between 1.5 and 150 µg/plate. The revertant colony counts in the solvent control group were within the historical in-house range of the respective tester strains. Significant increases (P ≤ 0.05) in revertant colony counts were noted in the positive control groups of 2-AA with presence of S9 and Dexon or SA without presence of S9 for all tester strains, indicating expected positive outcomes. No test article related increase in the revertant colony counts were noted in any tester strains at dose levels between 1.5 and 150 µg/plate. The slight but statistically significant changes (P ≤ 0.05) in the revertant colony counts noted at the dose levels between 1.5 and 150 µg/plate was considered a normal fluctuation within the normal in-house range. In summary, no cytotoxicity of KBP-5074 was noted at the dose levels between 1.5 and 150 µg/plate. No mutagenicity of KBP-5074 was noted in any tester strains at the dose levels between 1.5 and 150 µg/plate under the conditions of this study.
In vitro chromosomal aberration: No precipitate was noted in any dose formulations of KBP-5074 and no precipitate was noted when the formulations were adding to the cell cultures. Significant increases (P ≤ 0.05) in chromosomal aberration ratio compared with vehicle control group were noted in CP treated cells with presence of S9 (11%) and in MMC treated cells (15% and 60% for 4-hour and 24- hour treatment, respectively), indicating expected positive outcomes. No significant increase (P>0.05) in chromosomal aberration ratio compared with vehicle control group was noted in KBP-5074 treated cells with or without presence of S9, with the ratios no more than 2%. In conclusion, no genotoxicity of KBP-5074 was noted in the chromosomal aberration test at the concentrations up to 20 µg/mL for 4-hour treatment with absence or presence of S9 and up to 5 µg/ mL for 24 hours treatment with absence of S9 under the experimental conditions.
In vivo mouse micronucleus: No overt clinical signs of toxicity were observed in any male and female mice during the study. No significant increase in the ratio of PCE to total erythrocytes compared with vehicle control group was noted at all dose levels, indicating no bone marrow toxicity of KBP-5074 solid dispersion was noted at all dose levels in the test. A significant increase (P ≤ 0.05) in the incidence of micronucleus compared with vehicle control group was noted in both sexes of positive control group at 24 hours post-dose, indicating expected positive outcomes. No significant increase in the incidence of micronucleus compared with vehicle control group was noted in both sexes of KBP-5074 treated mice at 24 hours post-dose. A significant increase (P ≤ 0.05) in the incidence of micronucleus was noted in both sexes of high-dose level group of test article at 48 hours post-dose (Table 1). The increases were considered of no toxicological significance as the values were within the 3% negative control range published for CD-1 mice.
| Group | Number of animals | 24 hours post-dose | 48 hours post-dose | ||
| Number of Micronucleus Male/Female | Incidence of micronucleus Male/Female | Number of Micronucleus Male/Female | Incidence of micronucleus Male/Female | ||
| 1 | 5 | 1/0 | 0.1/0.0 | 2/2 | 0.2/0.2 |
| 2 | 5 | 5/5 | 0.5/0.5 | -/- | -/- |
| 3 | 5 | 2/1 | 0.2/0.1 | -/- | -/- |
| 4 | 5 | 1/0 | 0.1/0.0 | 9/11 | 0.9*/1.1* |
| 5 | 5 | 291/350 | 29.1*/35.0* | -/- | -/- |
Table 1: Micronucleus Test of KBP-5074 Orally Administered to Male and Female CD-1 Mice.
*P ≤ 0.05 vs.
Group1 vehicle control group.
Group 2, 3, 4 mice received oral doses of KBP-5074 at 60, 200 and 600 mg/kg, respectively.
Group 5 mice received Cyclophosphamide (Positive control);
“-”: blank.
Acute oral toxicity study in SD rats: No mortality was noted in any rats throughout the study. Treatment-related abnormal clinical observations including greenish feces, soft stool and soiled anal area were noted in 9/10 of males and 8/10 of females at 600 mg/kg approximately 3-8 hours after dosing on Day 1 and with consequent recovery from Day 2. A transient decrease in body weight gain was noted in treated animals as compared to the negative control group on Day 4, especially high females at 600 mg/kg (approximate 7.5% body weight loss) observed with gradual recovery from Day 8, which correlated with a decrease in food consumption at 600 mg/kg on Days 1-3 with gradual recovery from Day 4. No abnormal macroscopic findings were noted in any animal at terminal necropsy on Day 15. Based on these results, the maximum tolerated dose (MTD) of KBP5074 after a single oral administration to male and female rats was equal to or greater than 600 mg/kg.
4-Week oral toxicity study in SD rats: Rationale for the selection of dose levels employed in this study was based on the results of the 14-day oral gavage range finding study in rats (Supplementary data; Appendix A). Neither mortality nor overt clinical signs of toxicity were observed in control and KBP-5074 treated animals. Dose-related decreases in mean body weight and body weight gain were noted in female rats at 6 and 20 mg/kg/day when compared to vehicle controls. In male rats, a slight decrease was observed in mean body weight gain at the 20 mg/kg/day group. A significant decrease in food consumption was noted in female rats at 6 mg/kg/day and in both males and females at 20 mg/kg/day during Week 1. The above changes were reversible during thet reatment-free recovery period. Treatment-related changes in clinical chemistry parameters on Day 29 included increased alanine aminotransferase, blood urea nitrogen at 6 and 20 mg/kg/day groups, aspartate aminotransferase, alkaline phosphatase and potassium concentrations at 20 mg/kg/day group (Table 2). Decreases in total protein, albumin, creatinine, sodium and chloride levels were noted at 20 mg/kg/day group on Day 29 but these changes were reversible by Day 59. No significant changes in any clinical chemistry parameters were observed in male rats up to 20 mg/kg/day on Days 29.
| Group | Dose Level (mg/kg/day) | Number of rats | K+(mmol/L) Male | K+(mmol/L) Female | |
| 1 | 0 | 10 | Mean | 4.98 | 4.43 |
| SE | 0.10 | 0.13 | |||
| 2 | 2 | 10 | Mean | 5.12 | 4.43 |
| SE | 0.09 | 0.13 | |||
| 3 | 6 | 10 | Mean | 5.05 | 4.78 |
| SE | 0.15 | 0.19 | |||
| 4 | 20 | 10 | Mean | 5.17 | 5.06* |
| SE | 0.13 | 0.21 |
Table 2: Summary of plasma potassium concentrations in rats on Day 29.
*P ≤ 0.05 versus controls (Group 1).
A dose-related increase in plasma KBP-5074 concentrations were determined in male and female rats after oral administration at 2, 6 and 20 mg/kg/day (Supplementary data; Appendix B). Plasma levels of KBP-5074 appeared to be 2-3 times higher in the female rats. Pharmacokinetic analyses showed a dose-proportional increase in Cmax and AUC(0-24h) values of KBP-5074 on Days 1 and 28 between the range of 2 to 20 mg/kg/day (Figures 1-4, Table 3). There was no apparent drug accumulation after 28 days of consecutive dosing.

Figure 1: Cmax values of KBP-5074 in male rats on Days 1 and 28.
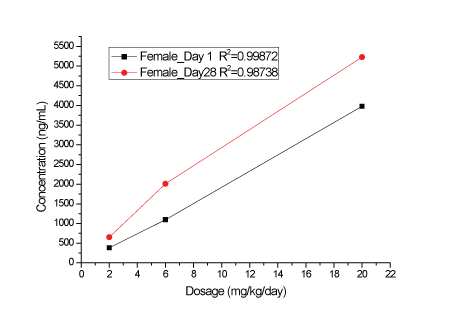
Figure 2: Cmax values of KBP-5074 in female rats on Days 1 and 28.
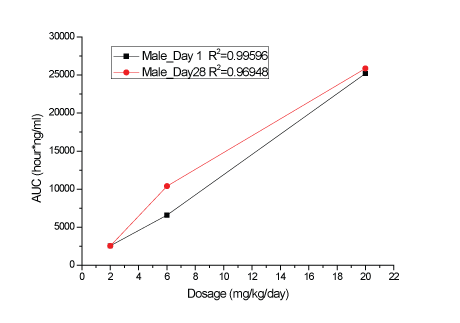
Figure 3: AUC(0-24h) values of KBP-5074 in male rats on Days 1 and 28.
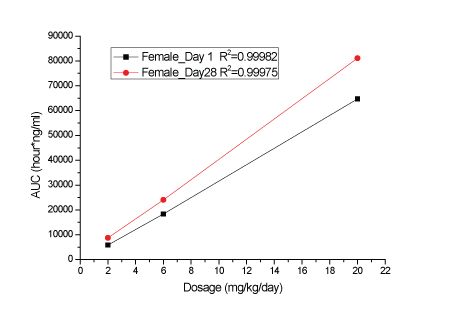
Figure 4: AUC(0-24h) values of KBP-5074 in female rats on Days 1 and 28.
| Dose (mg/ kg/day) | Date | Sex | Cmax(ng/mL) | AUC(0-24h) (h*ng/mL) |
AUC(0-24h) ratio |
| 2 | Day 1 | Male | 257 | 2552 | |
| Female | 383 | 5832 | |||
| Day 28 | Male | 353 | 2548 | ||
| Female | 653 | 8753 | |||
| 6 | Day 1 | Male | 698 | 6596 | 2.58 |
| Female | 1095 | 18384 | 3.15 | ||
| Day 28 | Male | 1327 | 10393 | 4.08 | |
| Female | 2010 | 24047 | 2.75 | ||
| 20 | Day 1 | Male | 2273 | 25191 | 9.87 |
| Female | 3980 | 64717 | 11.1 | ||
| Day 28 | Male | 3347 | 25858 | 10.2 | |
| Female | 5230 | 81093 | 9.26 |
Table 3: KBP-5074 Toxicokinetics of 4-Week Toxicity Study in Rats.
Increased kidney weights (relative to body and brain weight) were present in females dosed with 6 and 20 mg/kg/day and a dose-related increase in prostate weight relative to body and brain weight was present in males on Day 29. These changes were reversible without corresponding histopathological findings. Reversible hypertrophy of the adrenal zona glomerulosa was present at a slight to moderate severity in all the 20 mg/kg/day-treated male and female rats and with minimal severity in the 6 mg/kg/day-treated females. A non-reversible slight to moderate atrophy of the male mammary gland occurred in three males in the 20 mg/kg/day group and in one male from each of the 2 and 6 mg/kg/day groups. Atrophy of the male mammary gland is a rat-specific finding that is usually seen in response to hormonal alteration (decreased androgen or increased prolactin stimulation). The male rat mammary gland is uniquely sensitive to hormonal disruption and atrophy has been reported in response to antiandrogenic activity or hyperprolactinemia [11-13]. Atrophy of the male rat mammary gland may not be representative. It lacks clinical significance and is not considered to be clinically relevant. This supports other researchers’ hypotheses that the morphological changes of mammary gland observed in rats may not be expected in humans and other animal species [14]. No treatment-related toxicological findings were noted in any of the following parameters: clinical observations, ophthalmoscopic examinations, hematology, coagulation and urinalysis. In summary, the NOAEL of KBP-5074 was 6 mg/kg/day after 28 days of dosing in rats. The corresponding AUC(0-24h) and Cmax of KBP-5074 on Day 28 was 10393.35 ng*h/ml and 1326.67 ng/ml in males, or 24046.75 ng*h/ml and 2010.00 ng/ml in females, respectively (Table 3).
Acute oral toxicity study in beagle dogs: No mortality was noted in any animal throughout the study. No apparent abnormalities were observed in the three treatment groups throughout the study with the exception of emesis noted within 15 minutes after dosing in the female animal in Group 2 (60 mg/kg) and in a female animal in Group 3 (200 mg/kg) on Day 2 and two animals in Group 4 (600 mg/kg) on Days 2, 3 and/or 5; additionally, soft stool was noted in a male animal in Group 3 (200 mg/kg) on Days 2 and 3. No apparent abnormalities were noted in any of the following throughout the study: body weight, food consumption, body temperature, electrocardiogram, hematology, coagulation, or clinical chemistry. No gross lesions were found at scheduled necropsy on Day 15. In summary, no apparent toxicity was noted in any dog after administration of KBP-5074 solid dispersion via oral gavage at single doses of 60, 200, or 600 mg/kg. The MTD of KBP5074 was concluded to be equal to or greater than 600 mg/kg.
4-week oral toxicity study in beagle dogs: Rationale for the selection of dose levels in this study was based on the results of a 7-day oral gavage range finding study in dogs (Supplementary data; Appendix C). No mortality or clinical signs of toxicity were observed in any control or treated animals during the study. A slightly decreased trend in body weight gain was noted in animals after oral administration of KBP-5074 at 6 and 20 mg/kg/day between Day 7 to Day 28 and in female animals from Day 21 to Day 28. A gradual recovery in the changes of body weight was noted during the 4-week recovery period. An apparent decrease in food consumption was noted in male animals at 20 mg/kg/day from Day 13 to Day 27.These changes correlated with the decrease of body weights. No apparent changes in body temperature, electrocardiogram, urine analysis, ophthalmoscopic examination, hematology, coagulation and clinical chemistry parameters were noted in any animal throughout the study. There was no significant difference in plasma concentrations after oral administration of KBP-5074 to male and female dogs when compared to pre-dose (Day -6) and control groups (Table 4).
| Group | Dose Level (mg/kg/day) | Day -6 K+ (mmol/L) Male |
Day 29 K+ (mmol/L Male |
Day 57 K+ (mmol/L) Male |
Day -6 K+ (mmol/L) Female |
Day 29 K+ (mmol/L) Female |
Day 57 K+ (mmol/L) Female |
|
| 1 | 0 | Mean | 5.08 | 4.61 | 5.19 | 4.51 | 4.41 | 4.63 |
| SE | 0.09 | 0.03 | 0.41 | 0.12 | 0.07 | 0.12 | ||
| n | 6 | 6 | 2 | 6 | 6 | 2 | ||
| 2 | 2 | Mean | 5.03 | 4.64 | 5.11 | 4.44 | 4.25 | 4.47 |
| SE | 0.11 | 0.14 | 0.15 | 0.03 | 0.03 | 0.19 | ||
| n | 6 | 6 | 2 | 6 | 6 | 2 | ||
| 3 | 6 | Mean | 4.99 | 4.84 | 4.90 | 4.70 | 4.57 | 5.14 |
| SE | 0.15 | 0.08 | 0.02 | 0.13 | 0.13 | 0.20 | ||
| n | 6 | 6 | 2 | 6 | 6 | 2 | ||
| 4 | 20 | Mean | 4.90 | 4.83 | 4.96 | 4.68 | 4.64 | 4.97 |
| SE | 0.10 | 0.14 | 0.12 | 0.06 | 0.11 | 0.31 | ||
| n | 6 | 6 | 2 | 6 | 6 | 2 |
Table 4: Plasma Potassium Concentrations in Male and Female Beagle Dogs.
Plasma KBP-5074 concentrations after oral administration to male and female dogs at 2, 6 and 20 mg/kg/day on Days 1 and 28 were shown in Figures 5-7. No significant difference in the KBP-5074 plasma systemic exposure was observed between male and female dogs (Supplementary data; Appendix D). As a result, plasma KBP5074 data in males and females were pooled for the calculation of toxicokinetic parameters (Table 5). Mean Cmax and AUC(0-24h) values of KBP-5074 increased dose-proportionally with dose between the range of 2-20 mg/kg/day. No significant drug accumulation was observed after 28 days of consecutive dosing. The accumulation factors of KBP5074 were 0.83, 1.10 and 1.05 for 2, 6 and 20 mg/kg/day, respectively (Table 5).
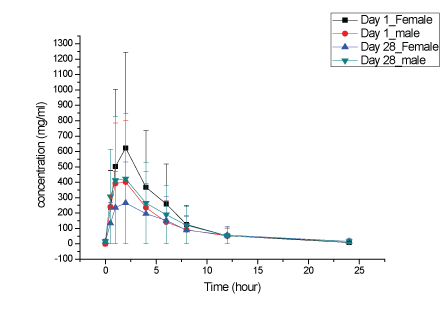
Figure 5: KBP-5074 plasma concentrations in male and female dogs at 2 mg/kg/day.
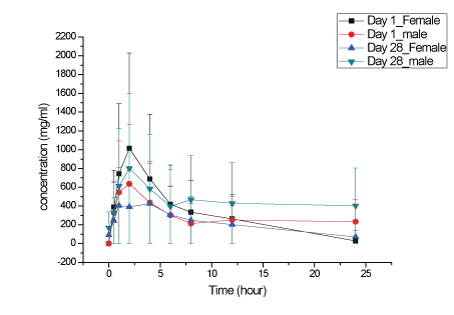
Figure 6: KBP-5074 plasma concentrations in male and female dogs at 6 mg/kg/day.
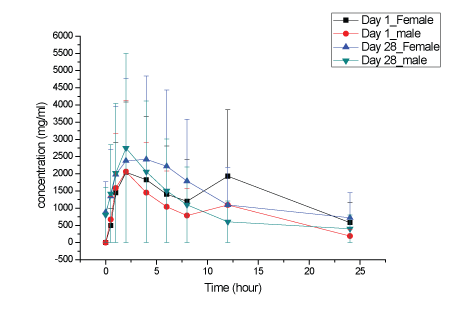
Figure 7: KBP-5074 plasma concentrations in male and female dogs at 20 mg/kg/day.
| Dose mg/kg/day | Time | Cmax (ng/mL) | AUC(0-24h) (h*ng/mL) | AI | Cmax ratio | AUC(0-24h) ratio | |
| 2 | Day 1 | n | 12 | 12 | |||
| mean | 530 | 3032 | |||||
| SD | 173 | 800 | |||||
| Day 28 | n | 12 | 12 | ||||
| mean | 364 | 2520 | 0.83 | ||||
| SD | 199 | 1232 | |||||
| 6 | Day 1 | n | 12 | 12 | |||
| mean | 885 | 7413 | 1.67 | 2.45 | |||
| SD | 309 | 3512 | |||||
| Day 28 | n | 12 | 12 | ||||
| mean | 759 | 8188 | 1.10 | 2.09 | 3.25 | ||
| SD | 499 | 6646 | |||||
| 20 | Day 1 | n | 12 | 12 | |||
| mean | 2770 | 27661 | 5.23 | 9.12 | |||
| SD | 1733 | 23440 | |||||
| Day 28 | n | 12 | 12 | ||||
| mean | 2700 | 29000 | 1.05 | 7.42 | 11.51 | ||
| SD | 1158 | 16637 |
Table 5: KBP-5074 TK Parameters of 4-Week Oral Toxicity Study in Beagle Dogs.
AI=(AUC(0-24h) D28/dosage)/(AUC(0-24h)D1/dosage);
Cmax ratio=Cmax/Cmaxlow dose;
AUC(0-24h) ratio=AUC(0-24h)/AUC(0-24h)low dose.
On Day 1, the mean AUC(0-24h) and Cmax ratios were 1:2.45:9.12 and 1:1.67:5.23, respectively. On Day 28, the mean AUC(0-24h) and Cmax ratio were 1:3.25:11.51 and 1:2.09:7.42, respectively. Slightly increased severity of lymphoid depletion in the thymus in a few animals at ≥ 6 mg/kg/day and nonspecific weight increases in the adrenal glands at ≥ 6 mg/kg/day were observed predominantly in females and were consistent with stress. Increased prostate weights (absolute and relative to body and brain weight) in males dosed at ≥ 6 mg/kg/day correlated with increased testes weights and degree of maturation and were concluded to be related to variations in sexual maturity and not related to KBP-5074. In summary, the NOAELs of KBP-5074 in male and female dogs were 6 and 2 mg/kg/day, respectively. The corresponding AUC(0-24h) and Cmax of KBP-5074 at 6 mg/kg/day for males on Day 28 were 3087 ng·h/ml and 287 ng/ml and 2 mg/kg/day for females on Day 28 were 2877 ng·h/ml and 460 ng/ml, respectively
Effect on central nervous system function in SD rats via oral gavage: The results showed that a single oral gavage dose of KBP-5074 solid dispersion to SD rats at doses of 1, 3 or 10 mg/kg did not have any drug-related effect on central nervous system function compared with control at 4, 24 and 48 h after dosing. The NOAEL of a single gavage of KBP-5074 solid dispersion administered to SD rats for CNS effect was ≥ 10 mg/kg (highest dose), suggesting that KBP-5074 did not appeared to have any potential CNS effects.
Effects on cardiovascular and respiratory function in conscious beagle dogs: No treatment-related statistical changes, trends of changes, or abnormality in individual data for these parameters were observed over the dose range compared to the pre-dose (-1 hour) and vehicle control values (Tables 6 and 7). Additionally, there was no gender-related difference in response observed due to the test article. The results showed that a single oral gavage dose of KBP-5074 solid dispersion to conscious beagle dogs at doses of 0.6, 3 or 15 mg/kg did not cause any drug-related effects on cardiovascular or respiratory system function. The NOAEL of KBP-5074 solid dispersion in cardiovascular and respiratory system function of conscious dogs was ≥ 15 mg/kg (top dose), suggesting that KBP-5074 demonstrated no potential cardiovascular or pulmonary effects.
| Dosage / Time point |
0 | 0.6 mg/kg | 3 mg/kg | 15 mg/kg |
| -1h | 71.4 ± 14.6* | 65.0 ± 15.2 | 70.9 ± 17.0 | 72.1 ± 10.8 |
| 0.5h | 75.1 ± 18.1 | 67.9 ± 16.9 | 72.8 ± 13.9 | 77.9 ± 12.2 |
| 1h | 67.3 ± 11.6 | 64.6 ± 11.3 | 69.8 ± 11.1 | 72.4 ± 5.3 |
| 2h | 75.1 ± 15.3 | 73.3 ± 14.0 | 74.9 ± 16.6 | 76.0 ± 12.0 |
| 3h | 71.5 ± 13.3 | 72.8 ± 13.9 | 66.0 ± 5.1 | 79.5 ± 17.7 |
| 4h | 73.8 ± 17.2 | 77.8 ± 18.1 | 81.8 ± 15.6 | 82.1 ± 19.7 |
| 6h | 74.6 ± 14.0 | 76.5 ± 18.2 | 76.0 ± 18.6 | 82.3 ± 18.3 |
| 8h | 91.6 ± 11.3 | 92.0 ± 10.0 | 88.1 ± 15.1 | 92.9 ± 8.7 |
| 12h | 95.1 ± 10.6 | 88.0 ± 14.9 | 87.9 ± 13.2 | 96.5 ± 10.9 |
| 24h | 77.3 ± 25.9 | 73.5 ± 14.2 | 77.0 ± 18.4 | 72.0 ± 14.7 |
Table 6: Summary of KBP-5074effects on heart rates in the dogs.
*Beats/minute, ( X SD ± , N=8).
| Dosage Time point | 0 | 0.6 mg/kg | 3 mg/kg | 15 mg/kg |
| -1 h | 92.22 ± 10.13* | 92.93 ± 6.22 | 95.41 ± 5.32 | 96.49 ± 6.38 |
| 0.5 h | 91.75 ± 6.93 | 90.58 ± 4.12 | 90.05 ± 6.18 | 90.38 ± 5.83 |
| 1 h | 91.53 ± 5.95 | 90.09 ± 6.50 | 89.87 ± 7.42 | 90.97 ± 5.35 |
| 2 h | 96.71 ± 10.55 | 97.99 ± 7.99 | 91.98 ± 5.49 | 98.08 ± 6.87 |
| 3 h | 94.07 ± 9.89 | 92.83 ± 8.41 | 95.37 ± 5.10 | 90.18 ± 5.13 |
| 4 h | 95.92 ± 5.32 | 93.32 ± 11.09 | 93.54 ± 6.80 | 97.66 ± 9.49 |
| 6 h | 89.71 ± 9.16 | 92.76 ± 6.67 | 91.90 ± 5.66 | 91.66 ± 10.99 |
| 8 h | 93.81 ± 6.91 | 92.58 ± 6.78 | 94.14 ± 5.50 | 94.74 ± 10.21 |
| 12 h | 88.46 ± 10.28 | 93.33 ± 6.20 | 95.30 ± 6.92 | 94.67 ± 11.13 |
| 24 h | 94.57 ± 9.73 | 94.14 ± 9.21 | 94.33 ± 9.64 | 95.21 ± 4.78 |
Table 7: Summary of KBP-5074 effects on mean arterial blood pressures in the dogs.
*mmHg ( X SD ± , N=8).
In vitro hERG assay: KBP-5074, at the concentrations tested (1- 30 µM), inhibited the electric currents passing through cloned hERG potassium channels stably expressed in Chinese Hamster Ovary (CHO) cells. The average inhibition at the highest test concentration was 34.2% and the IC50 value was ≥ 17 µM.
In vitro genotoxicity studies demonstrated that KBP-5074 was neither mutagenic nor clastogenic. In addition, mouse micronucleus test of KBP-5074 showed no significant increase in the incidence of micronucleus was observed at 24 hours post-dose in the male and female mice when compared with control animals. However, a significant increase (P ≤ 0.05) in the incidence of micronucleus was noted at the high dose group in both sexes at 48 hours post-dose. The increases were considered of no toxicological significance because 3% increase was within the range of in-house historical control data and the published mean spontaneous micronucleus frequencies in CD-1 mice [15]. Based on these in vitro and in vivo test results, it was concluded KBP-5074 would not pose a genotoxic or carcinogenic hazard to humans.
Acute oral toxicity of KBP-5074 at doses up to 600 mg/kg showed no overt clinical signs of toxicity in SD rats and Beagle dogs. It was concluded that the maximum tolerated dose (MTD) of KBP-5074 was equal to or greater than 600 mg/kg in rats and dogs. In the pivotal INDenabling 4-week oral rat study, no significant clinical signs of toxicity were observed in male and female rats. No significant changes in any clinical chemistry parameters were observed after oral administration of KBP-5074 at doses up to 20 mg/kg/day in the male rats on Day 29. However, elevation of plasma potassium concentrations was observed in the female rats at 20 mg/kg/day on Day 29. The gender difference on potassium levels was because the plasma KBP-5074 concentrations were 2-3 times higher in female than male rats after oral administration. In the 4-week dog study, no significant clinical signs of toxicity, changes in body temperature, electrocardiogram, urine analysis, ophthalmoscopic examination, hematology and clinical chemistry parameters (including potassium) were noted in any animals at doses up to 20 mg/kg/day. Plasma concentrations of KBP5074 in male and female dogs were equivalent to those observed in male rats after oral administration at the same doses and significantly lower than those in the female rat at the corresponding dose levels. No gender difference was observed in the toxicokinetic parameters of KBP-5074 in male and female dogs after oral administration at doses up to 20 mg/kg/day.
Eplerenone is the second (after spironolactone) oral aldosterone antagonist available for the treatment of arterial hypertension and heart failure. Treatment with eplerenone has been associated with decreased blood pressure and improved survival in patients with heart failure. However, the most common and undesirable side effect of spironolactone, i.e. hyperkalemia, was also reported with eplerenone [16]. Recently, three nonsteroidal MRAs have reportedly demonstrated an improved therapeutic index for hyperkalemia in comparison to steroidal MRAs. Esaxerenone (CS-3150) is another oral non-steroidal MRA currently undergoing clinical trials for the treatment of essential hypertension and cardiorenal diseases [17,18]. In DSS rat model of hypertension, CS-3150 prevented the increase in SBP in a dose-dependent manner without hyperkalemia [18]. Finerenone is a third generation MRA which has shown a significant reduction in UACR without hyperkalemia in patients with CKD [19]. It is noteworthy that the increased in plasma potassium levels was observed only in female rats after oral administration of KBP-5074 at 20 mg/kg/day for 4 weeks. There was an apparent gender difference in the TK/metabolism of KBP-5074 in the rats. The Cmax and AUC were significantly higher in females than males. The changes in plasma potassium concentrations were reversible after a treatmentfree recovery period.
The CNS effect of KBP-5074 was assessed by a single oral gavage dose of KBP-5074 to SD rats at doses of 1, 3 and 10 mg/kg. The results showed that a single gavage dose of KBP-5074 at doses up 10 mg/kg did not result in any drug-related effects on CNS functions compared to the control at 4, 24 and 48 h after dosing. The NOAEL was determined to be ≥ 10 mg/kg. The effect of KBP-5074 on the cardiovascular and respiratory systems was assessed by a single oral gavage dose of KBP5074 to conscious Beagle dogs at doses of 0.6, 3 and 15 mg/kg. Results showed that a single oral gavage of KBP-5074 to conscious Beagle dogs at doses up to 15 mg/kg did not cause any drug-related effects on cardiovascular and respiratory system functions and the NOAEL was concluded to be ≥ 15 mg/kg. The IC50 of KBP-5074 on in vitro hERG assay was ≥ 17 µM, which was ≥ 1000-fold higher than its in vitro antagonistic activity (IC50: 2.7 nM) on recombinant human MR [1]. Redfern [20] reviewed the relationship between preclinical cardiac electrophysiology and torsade de pointes for a broad range of drugs and concluded that safety margins >30- to 100-fold would be adequate in drug development.
In summary, it was concluded that the preclinical safety profile of KBP-5074 would support the First-in-Human (FIH) study based on the results of the above studies. KBP-5074 induced toxicities were dose-dependent and reversible after cessation of treatment. The first proposed clinical study would be a Phase 1, single ascending dose (SAD) study in healthy subjects to evaluate the safety, tolerability, and pharmacokinetics of KBP-5074 following escalating single oral dose administration of KBP-5074. The proposed clinical doses for this SAD study were 0.5, 1.0, 2.5, 10 and 20 mg. The NOAEL of KBP-5074 in male and female rats was 6 mg/ kg/day in the 4-week study, which translated to a human equivalent dose (HED) of 0.97 mg/kg. The NOAEL of KBP-5074 in male and female dogs in the 4-week study were determined to be 6 and 2 mg/kg/day, respectively. The corresponding human equivalent dose (HED) was 3.33 and 1.11 mg/kg, respectively. Assuming the body weight of each subject averaged 60 kg each and using the conversion factors (Km) recommended by the FDA Guidance for Industry [21], the margin of safety for the proposed clinical dose of 0.5 to 20 mg would range from 2.9- to 115-fold, using the HED of 0.97 mg/kg derived from the 4-week rat study. The safety margins of KBP-5074 would be even higher based on the 4-week dog study because the NOAEL and HED values were higher than those derived from the 4-week rat study
Preliminary results of this paper were presented at the Society of Toxicology annual meeting in Texas on March 11, 2018. This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors. The authors declare no conflict of interest.
Supplementary information (Appendices A, B, C and D) to this article can be found along with this file.
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Chow CP, Liu JR, Tan XJ, Yang F, Huang ZH (2018) Preclinical Development of KBP-5074, a Novel Non-Steroidal Mineralocorticoid Receptor Antagonist for the Treatment of Cardiorenal Diseases. J Drug Res Dev 4(2): dx.doi.org/10.16966/2470-1009.143
Copyright: © 2018 Chow CP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access