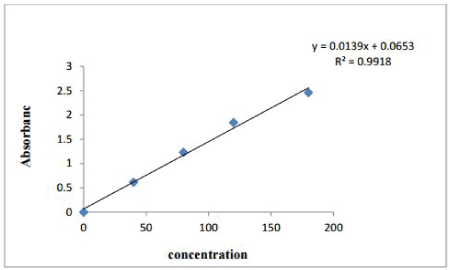
Figure 1: Lambert Beers plot for model drug amoxicillin


Sunil Kumar Bajpai* Farhan Ferooz Shah Manjula Bajpai Mamta Jadaun Pooja Jyotishi
Polymer Research Laboratory, Department of Chemistry, Govt. Model Science College, Jabalpur (MP) - 482001, India*Corresponding author: Sunil Kumar Bajpai, Polymer Research Laboratory, Department of Chemistry, Govt. Model Science College, Jabalpur (M.P)-482001, India, Tel: 09993220651; E-mail: sunil.mnlbpi@gmail.com
Objective: In the present work, a fast dissolving film has been developed, that consists of milk protein Casein and polysaccharide Sodium alginate. The films were characterized and investigated for their dissolution properties. Finally, films have been loaded with model drug Amoxicillin and investigated for drug release behavior.
Significance: The films have potential to be used for delivery of those drugs that are required for immediate relief to the patients. The polymers, used, are totally edible and non-toxic.
Methods: The films were prepared by the physical blending of the two polymers, namely casein and alginate. The dissolution/disintegration properties were investigated by putting a pre-weighed piece of the film in artificial saliva. The weight loss was recorded periodically. The films were also characterized by FTIR, XRD, and SEM analysis. Finally, the release kinetics of drug Amoxicillin was also studied.
Results: The FTIR spectra of the drug and drug-loaded film revealed the presence of drug in the film. SEM images indicated granular surface texture of the films. The Tensile Strength of the films lied between 1.25 to 1.36 MPa, suggesting no appreciable change on varying composition of films. The dissolution time of the films was found to be in the range of 460 to 510 seconds, depending upon the mutual concentration ratio of casein and alginate. The release of drug Amoxicillin was found to follow ‘zero order kinetics’ which is the most desirable for best therapeutics.
Conclusions: These films bear potential to be used for delivery of fast relieving drugs through oral mucosa route.
Casein; Alginate; Oral mucosa; Fast dissolving film; Physical blending
Orally dissolving film (ODF) may be described as a small strip, composed of water soluble polymers and a bioactive ingredient that has been uniformly dispersed in the film matrix. Indeed, a number of synonym terms, such as oral film, thin-film, wafer, oral strip, oral thin film and oral soluble film, etc. are frequently used [1,2,3]. According to European Medicine Agency (EMA), they are designated as orodispersible film. When this film is placed on the tongue, it readily disintegrates and dissolves to release the entrapped drug that is subsequently absorbed via oromucosal route [4]. It is reported that buccal mucosa has almost 4000 times greater permeability than that of skin [5]. The delivery of drug through oral mucosa has so many advantages such as direct delivery of the drug into the blood stream, which avoids not only its exposure to the harsh conditions of gastric environment but it also eliminates its first pass metabolism in the liver [6].
In order to prepare ODF, water soluble polymers with excellent film forming property, are generally used [7]. There has been huge number of reports available in the literature, describing use of hydrophilic polymers to prepare FDF for the purpose of enhancement in the oral bioavailability of some drugs. For example, Allam and Fetih [8] have prepared FDF, composed of hydroxypropyl methyl cellulose E15 and methyl cellulose and loaded them with entrapped metoprolol tartrate-loaded niosomes. Similarly, Krull et al. [9] have reported fast release of griseofulvin, a model poorly water-soluble drug, from pullulan based fast dissolving films. In another study by Lai and co-workers [10] the delivery and oral bioavailability of quercetin nanocrystals was enhanced using the fast dissolving film composed of maltodextrins as a film forming material and glycerin as plasticizer. Recently, Ganduri et al. [11] have reported pullulan based FDF and studied effect of concentration of Pullulan on the properties of the films. It was found that increase in the concentration of polymer in the film caused an enhancement in their disintegration and dissolution times.
Indeed, delivery of a drug through oromucosal route using fast dissolving films is an interesting and significant approach. However, since the film is dissolved on the surface of the tongue, the polymers used for film formation essentially need to be totally non-toxic [12]. Therefore, use of natural polysaccharides as well as food proteins is a better option than the synthetic polymers [3]. In the light of the above argument, we hereby report Casein/Alginate based fast dissolving film (FDF) for effective release of a model drug amoxicillin.
Casein, a milk protein, consists of around 93% of the total protein content of the milk [13]. Casein is composed of four phosphoproteins, named as αS1-, αS2-, β-, and κ-casein, with their respective weight proportions as 4:1:4:1 [14]. Being non-toxic and biocompatible, casein has frequently been used in drug delivery applications [15]. Due to excellent biocompatibility and biodegradability, sodium alginate has been widely employed in a number of applications such as edible films and coatings [16], wound dressings [17], in food industry [18], drug delivery [19], bone tissue engineering [20] etc. Most recently, Mooney and Lee [21] have reviewed biomedical applications of alginates. Alginate is made up of blocks of (1-4)-linked β-D-mannuronic acid (M) and α-L-guluronic acid (G) monomers, arranged as MMMM, GGGG, or MGMGMGMG [22].
SA (molar mass of 150,000 Da and M/G ratio of 1.62, HiMedia Chemicals, Mumbai, India), casein powder (Cas; alkali soluble, with purity of 99.6%, Research Lab, Pune, India), Other chemicals such as sodium hydroxide, sodium dihydrogen phosphate, were obtained from Central Drug House; Mumbai, India. The antibiotic drug amoxicillin was purchased from local medical shop (Batch No: AEH6017). The double distilled water was used throughout the investigations.
Preparation of Sodium Alginate/Casein uncross-linked films: Sodium alginate/Casein films were prepared by dissolving sodium alginate (Alg) and casein (Cas) in 0.5 % solution of NaOH in different weight ratios. In brief, to definite volume of 0.5% NaOH solution, Alg and Cas were dissolved in different proportions, under mild stirring, and the final volume of the solution was made to 15mL. The film forming solution was poured in to Petri plate (Borosil, id mm) and placed in electric oven (Temp star, India) for a period of 6h at 60°C. The films, thus formed, were peeled off carefully and kept in a dust-free chamber for further use. In all we prepared six film samples, in which %weight ratios of Alg: Cas were 80:20, 70:30, 60:40, 50:50, 40:60 and 30:70 respectively. The film samples were designated as MDF(x:y), where the numbers x and y are the weight % of Alg: Cas in the film forming solution.
Preparation of Amoxicillin loaded films: The antibiotic drug Amoxicillin (Amx) loaded films were prepared by the direct addition of amoxicillin in the film forming polymer solution. Two types of drug loaded films were prepared. (1) Film samples having different weight ratios of Alg: Cas but same concentration of amoxicillin and (2) Film samples having the same composition but loaded with different concentrations of amoxicillin.
In order to prepare first type of films, definite quantity of Amx was added under mild stirring into the three film forming solutions, having weight ratios of Alg: Cas as 70:30, 50:50 and 30:70, and poured into Petri plates. After putting in electric oven at 60°C for a period of 2h, the films formed were peeled off and kept in a vacuum chamber for further use. These films were designated as ODF (70:30)48, ODF(50:50)48 and ODF(30:70)48 respectively. Here, each film contained 48 mg of drug Amx per gram of film. In order to prepare the second type of films, film forming solution, having Alg: Cas ratio of 70:30 was prepared and divided in to three parts, followed by addition of varying quantities of drug Amx. These solutions were stirred and poured in to Petri plates, and finally transformed in to films by solvent evaporation as described above. These films were designated as ODF (70:30)31, ODF (70:30)48, ODF (70:30)66 and ODF (70:30)91 respectively. The experiments for each sample were done in triplicate. The compositions of various plain and drug-loaded films, prepared, are given in (Table 1).
| Sample | Sodium Alginate (mg) | Casein (mg) | Amount of Drug (mg) |
| ODF(80:20) | 800 | 200 | - |
| ODF(70:30) | 700 | 300 | - |
| ODF(60:40) | 600 | 400 | - |
| ODF(50:50) | 500 | 500 | - |
| ODF(40:60) | 400 | 600 | - |
| ODF(30:70) | 300 | 700 | - |
| ODF(70:30)48 | 700 | 300 | 48 |
| ODF(50:50) 48 | 500 | 500 | 48 |
| ODF(30:70) 48 | 300 | 700 | 48 |
| ODF(70:30) 31 | 700 | 300 | 31 |
| ODF(70:30) 48 | 700 | 300 | 48 |
| ODF (70:30) 66 | 700 | 300 | 66 |
| ODF (70:30) 91 | 700 | 300 | 91 |
Table 1: Composition of various plain and drug-loaded films
The Fourier transform infrared (FTIR) spectra of films were recorded with an FTIR spectrophotometer (Shimadzu, 8400, Japan) using KBr. In order to investigate the surface morphology of the plain and drug-loaded films, SEM images were determined by Hitachi: S-4700 (New Jersey, USA) operating at an acceleration voltage of 15kV. Surface morphologies were imaged at different magnifications. The X-ray diffraction (XRD) method was used to measure the crystalline nature of the films. These measurements were carried out on Rigaku Miniflex 300/600. The diffractogram was recorded in the range of 2 from 3 to 500 at the speed rate of 2 degree/min.
The tensile strength of all the six samples, namely ODF (80:20), ODF (70:30), ODF (60:40), ODF (50:50), ODF (40:60) and ODF (30:70) was determined. In addition, effect of presence of the plasticizer glycerol in the film on the tensile property was also investigated. For this, 0.5mL of glycerol was added to the film forming solution for the sample ODF (70:30) and the resulting film was designated as ODF (70:30)0.5. The mechanical properties of the films were determined using Unique Tensile Tester (UTM, Universal Tensile Tester, and Pune, India) according to the standard testing method ASTM D882-97 [23]. After preconditioning the films at 50% relative humidity (RH), films were cut into 5 mm × 35 mm strips with a razor blade; then, the strips were allowed to equilibrate at ambient humidity overnight before mechanical testing. The measurements were made at 25°C under the constant humidity of 50%. The samples were cut into strips, each of width 5 mm and length 50 mm. The initial grip separation and mechanical crosshead speed were set at 25 and100 mm per min, respectively. The various formulae used are shown as below:
Tensile strength (σ)=Force or load (F)/MA
Where, F is the maximum load and MA is the minimum cross-sectional area of the film specimen.
Results were converted to mega Pascal units (MPa).
The artificial saliva (AS) was prepared as described elsewhere [24]. In brief, Sodium chloride-0.844 g; Potassium chloride-1.2 g; Calcium chloride dihydrate-0.193g; magnesium chloride hexahydrate-0.111 g; potassium phosphate dibasic-0.342 g. These ingredients were added one by one to 500 mL of distilled water and then the volume was made up to 1000 mL using the same. The pH was adjusted with 0.1N hydrochloric acid to 5.7
The disintegration time is the time when a film starts to break or disintegrate and the time at which the film is completely dissolved is called dissolution time [25]. The films were cut into 1 × 1 cm and the in vitro disintegration and dissolution time of all film formulations was determined visually in a glass dish of 20 mL of artificial saliva (AS) with swirling every 20 seconds. Disintegration and dissolution time was measured.
The pre-weighed piece of drug-loaded film was placed in 20 mL of artificial saliva (i.e. AS) at 37°C. After regular time-intervals, the amount of drug released was determined spectrophotometrically at 272 nm. The quantity of drug was calculated using Lambert–Beer’s plot obtained for drug solutions of known concentrations. In order to obtain the plot for drug, standard drug solutions were prepared in the distilled water in the concentration range of 50 to 200 mg per dL and their absorbance was recorded. The Lambert Beers law is shown in Figure 1.

Figure 1: Lambert Beers plot for model drug amoxicillin
In order to investigate the effect of sweetener, saliva stimulating agent and surfactant on the dissolution of film, film sample [ODF (70:30)] was selected for study. We prepared film samples by adding different amounts of each of sweetener, saliva stimulating agent and surfactant and compared their dissolution with the plain sample taken as control.
It is well established fact that casein consists of four types of constituents, named as αS1-, αS2-, β-, and κ-casein. The various polar groups, present in casein molecules, have fair ability to form inter-molecular hydrogen bonding [26]. Likewise, sodium alginate also contains various polar groups such as –OH and –COOH, present along mannuronic and guluronic residuals. When, the two compounds, i.e. casein and alginate are dissolved in water, these polar groups interact with each other and establish H-bonding interactions. On evaporation of solvent, blend, consisting of Cas and Alg, is formed. Similarly, Casein is known as to have random coil nature and possess fair tendency to form hydrogen bonding. As there are no cross-linking agents used in the preparation of films from the polymers Cas and Alg, the film must possess only physical type of attractive forces as mentioned above. The films, produced, were almost opaque and there was a gradual shift from pale yellow to brown the alginate content increased in the films. As an illustration, Figures 2a-c shows optical images of three ODF (% Alg: % Cas) strips, namely ODF(40:60), ODF(60:40) and ODF(80:20) respectively. It can be seen that as the Alg content increases, the color of the film shifts from pale yellow to brown.
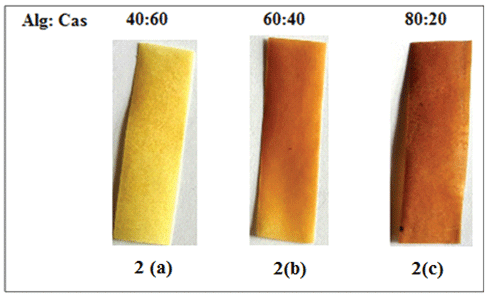
Figure 2: Optical images of ODF (% Alg : % Cas) strips, (a) ODF(40:60), (b) ODF(60:40), (c) ODF(80:20)
The antibiotic drug Amoxcillin (Amx) loaded films were prepared by adding a pre-determined quantity into the polymerization mixture. A calculated amount of drug was added into the reaction mixture for all the film samples. The per gram drug loading (PGDL) was determined as follows: The drug-loaded film was washed with distilled water for a definite time and the amount of drug leached out of the film was measured spectrophotometrically at 272 nm. The actual drug loading was calculated using the following formula:
(PGDL) Amount of drug/g film=(W1 -W2 )/W3
Where, W1 =Amount of drug in the feed mixture
W2 =Amount of drug leached out in washing
W3 =Weight of the film
FTIR analysis: From infrared spectroscopic analysis of free casein Figure 3a, the functional group assignments meant for –NH2, NH, C=O, OH and C-O bond vibrations are clear at 3471, 3132, 1672, 3230 and 1149 (cm-1), respectively. Similarly the vibrational frequencies in case of free sodium alginate Figure 3b are 1599 cm-1 for C=O, 3309 cm-1 for –OH, 937 cm-1 for C-O-C and 1151 cm-1 for C-O bond. The linking of sodium alginate with casein is designated by the appearance of stretching vibrations of NH2 , NH, C=O, -OH, C-O-C, C-O, respectively at 3466 cm-1 , 3137 cm-1, 1646 cm-1, 3466 cm-1, 937 cm-1 and 1096 cm-1 Figure 3c. The observed changes are indicative of effectiveness of hydrogen bonding (or non-covalent interactions) by the share of lone pair of electrons at the respective sites [27].
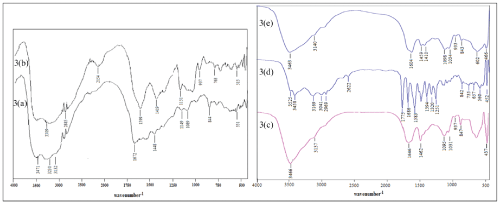
Figure 3: FTIR spectrum of (a) casein, (b) sodium alginate, (c) Plain film ODF (70:30), (d) Amoxicillin, (e) Amoxicillin loaded film ODF (70:30)48
The characteristic vibrational peak at 657 cm-1 (C-S) in Figure 3d of drug amoxicillin can also be identified in the above linked polymer stretching at 602 cm-1 (C-S) Figure 3e along with vibrational frequencies of 3463 cm-1 (NH2 ), 3140 cm-1 (NH), 1604 cm-1 (C=O), 3463 cm-1 (OH), 933 cm-1 (C-O-C). Thus, confirms the loading of drug amoxicillin in the above linked polymer.
SEM analysis: Scanning electron microscopy provides useful information about the texture of film. The Alg/Cas films, prepared at different compositions, were having rough surfaces. The SEM images of a representative sample ODF (70:30), at the magnifications of 1000, 5000 and 15000X are shown in Figures 4a-c respectively. The surface appears to have course and granular texture, with particles having a high length to width ratio. The SEM images of drug-loaded film sample ODF (70:30)48, at same magnifications are also shown in Figures 4d-f respectively. It may be noticed that there is not much change in the surface texture due to loading of amoxicillin.
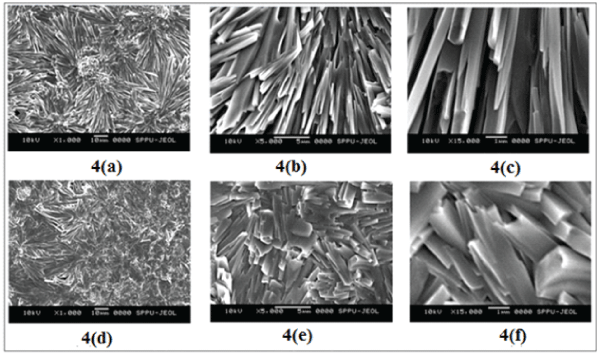
Figure 4: SEM images of ODF (70:30), at the magnifications of (a) 1000 X, (b) 5000 X (c) 15000 X and drug-loaded film sample ODF (70:30)48 at the magnifications of (d) 1000 X, (e) 5000 X (f) 15000 X
XRD analysis: The crystalline nature of the drug-loaded formulation was determined by XRD analysis, carried out for the pure drug, plain polymer ODF (70:30) and the drug-loaded polymer ODF (70:30)48, as shown in Figures 5a-c respectively. The powder XRD patterns of pure amoxicillin, as shown in (a), exhibited a number of peaks at 2θ values of 12.2°, 15.3°, 16.3°, 18.2°, 19.1°, 26.6°, 28.6°. Almost similar peaks have also been reported by Murthy NV et al. [28]. The XRD pattern of plain polymer ODF (70:30), shown in (b) shows semi-crystalline nature. In the film matrix, the strong intermolecular hydrogen bonding results in a intense broad peak at 22° , corresponding to reflections through (200) plane of poly (mannuronate) as also reported by P. Sundarrajan et al. [29]. Finally, the diffraction pattern of drug-loaded polymer ODF (70:30)48, as shown in (c), suggests predominance of broad peak observed due to alginate as mentioned earlier. However, the sharp diffraction peaks, observed in the diffractogram of pure drug, seem to be almost suppressed, may probably be due to predominance of amorphous alginate and gelatin, and also due to small concentration of drug.
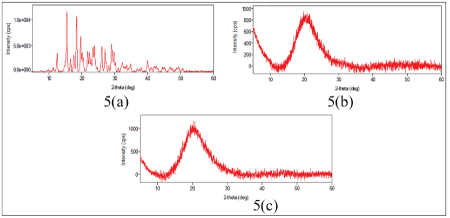
Figure 5: XRD patterns of (a) Pure Amoxicillin (b) ODF (70:30) (c) Amoxicillin loaded film sample ODF (70:30)48
Tensile strength of films: Tensile strength of all the six samples, prepared, namely ODF(80:20), ODF(70:30),ODF(60:40), ODF(50:50), ODF(40:60) and ODF(30:70) are shown in Figure 6. It may be seen that TS of all the six samples lie in the range of 1.25 to 1.36 MPa, thus indicating that there is no appreciable variation in TS of the films due to change in Alg : Cas ratio. However, the TS of the sample ODF(70:30)0.5, falls to 0.48 MPa due to addition of 0.5mL of the plasticizer glycerol. This may be attributable to the fact that glycerol molecules occupy the space between the alginate and casein chains, thus reducing the electrostatic interactions to a great extent. This, finally, reduces the TS of the film.
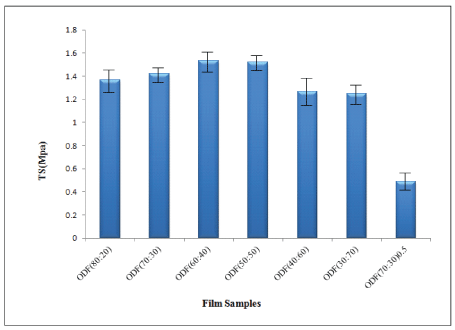
Figure 6: Variation in TS of all six film samples and glycerol loaded film sample
The dissolution time of an ODF is a major parameter to control the delivery of entrapped bioactive material. The faster is the dissolution of film, the greater will be the amount of drug released. In the present work, the dissolution times of various film samples was found to vary from 460 s to 510 s as the % content of casein in the film increased from 20 to 50 (Table 2).
| Sample | Disintegration Time (sec) | Dissolution Time (sec) |
| ODF(80:20) | 65 | 460 |
| ODF(70:30) | 67 | 475 |
| ODF(60:40) | 70 | 510 |
| ODF(50:50) | 70 | 510 |
| ODF(40:60) | 75 | Not dissolved |
| ODF(30:70) | 75 | Not dissolved |
Table 2: Variation in disintegration and dissolution time due to increase in % content of casein in the films
In addition, the samples with % Cas contents of 60 and 70 did not dissolve. This may be explained on the basis of the fact that casein has poor solubility in the saliva medium of pH 6.4, and therefore, as the Cas content in the film increase, the dissolution time increases. In addition, the samples with % Cas content of 60 and 70 do not dissolve, due to insolubility of casein in the pH of saliva. Hence the film samples ODF(40:60) and ODF(30:70) are not suitable for the oral delivery through orolomucosa. Here, it is also noticeable that since Alg has fair solubility, its lower content in the film enhances the dissolution time. Finally, the disintegration time of the films also increase with Cas content in the films. The films with % Cas contents from 20 to 70 showed disintegration times in the range of 65 to 75 s.
All the film samples studied were found to disintegrate. The increase in the disintegration time could be attributable to the relatively greater number of interactions among polar groups of casein molecules. In a study, Panchal et al. [30] have also reported almost similar trends for Ropinirole Hydrochloride loaded fast dissolving films, composed of Pullulan. The visual observations of dissolution and disintegration times for the sample ODF (60:40) at various time intervals are shown in Figure 7.
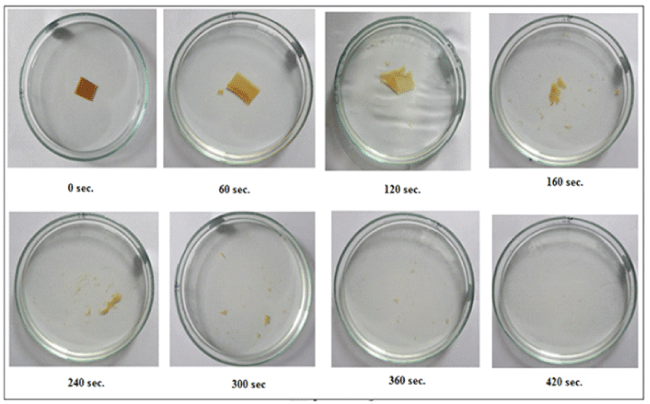
Figure 7: Visual observations of dissolution and disintegration times for the sample ODF (60:40) at various time intervals.
Effect of drug concentration: A film which is suitable for buccal drug delivery “should be flexible, elastic, soft yet adequately strong to withstand breakage due to stress from mouth activities [31]. For controlled drug release purposes, an ideal buccal film should have three properties: (i) it should maintain its position in the mouth for a desired duration, (ii) it should release a drug in a required controlled fashion, and (iii) it should provide drug release in a unidirectional way towards the mucosa. The release of drug Amoxicillin from the samples ODF (70:30)31, ODF(70:30)48, ODF(70:30)66, and ODF(70:30)91 was carried in AS of pH 6.4 as shown in Figure 8. The total amount of drug released from the film samples was 30.76, 47.86, 65.48 and 90.42 mg/g of film respectively.
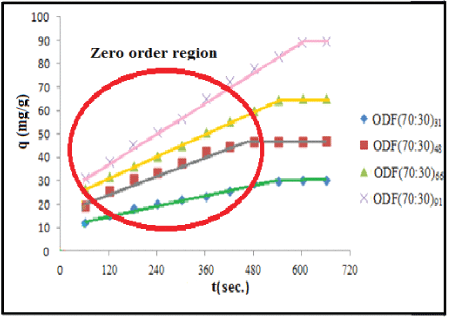
Figure 8: Dynamic release of drug Amx from the samples ODF (70:30)31, ODF (70:30)48, ODF (70:30)66, and ODF (70:30)91 in the PBS of pH 6.4
It is clear that as the amount of drug in the film increases, the amount of drug released at particular time also increases. This is likely due to the fact that higher concentration gradient is produced across the interface for the films with higher drug content. A close look at the profiles also reveals that after initial phase, the amount of drug released at different time intervals increases linearly with time. In other words, for a particular sample, drug is released at the same rate .This implies that for all the film samples studied, a “zero order” kinetics is followed. The “zero order region” has been encircled with red as can be seen in the above Figure 8. The encircled area contains linear plots for all the samples studied.
In order to determine the rate constant k0 , the linear portions, encircled in the above Figure 8, the following “Zero order” equation was employed :
q=k0t
Where, q is the amount of drug released at time ‘t’ and k0 is zero order rate constant . The slopes of the linear plots, displayed in Figure 8, were used to calculate first order rate constants, k0 , which were found to be 6.582, 4.734, 3.480 and 2.134 mg/g/s for the samples ODF(70:30)31, ODF(70:30)48, ODF(70:30)66, and ODF(70:30)91 respectively (Table 3). Here, it is noteworthy that a ‘zero order’ release is always the most desirable as it provides the drug at the target site at the same rate throughout. In addition, it also minimizes or almost eliminates the swings in drug concentrations.
| Sample | k ( mg/g /s)0 |
| ODF(70:30) 31 | 6.582 |
| ODF(70:30) 48 | 4.734 |
| ODF(70:30) 66 | 3.480 |
| ODF(70:30) 91 | 2.134 |
Table 3: First order constants for the release of Amoxycillin from various film samples at 37°C
The ‘zero order release’ from the fast dissolving films can be explained as follows: Figure 9 shows a drug-loaded ODF sample in contact with the surface of tongue. As the film begins to dissolve from its bottom, the drug is released and penetrates the mucus lining and enters the blood stream. As the dissolved part leaves away, fresh layer comes in contact with surface of tongue and undergoes dissolution with simultaneous release of drug. In this way drug is released from the dissolving film at the same rate and a ‘zero order’ is achieved. The occurrence of ‘zero order release’ in fast dissolving films is a widely reported phenomenon. For example, in a study by Abu El-Enin and co-worker [32] have reported a ‘zero order ‘ release of Metoclopramide hydrochloride from hydroxyl propyl methyl cellulose based fast dissolving film. Similar results have also been reported elsewhere [33].
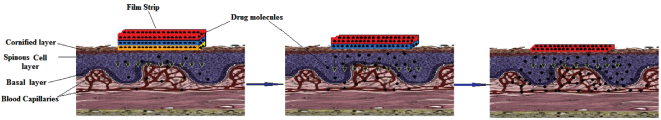
Figure 9: Scheme showing zero order release from the fast dissolving film
Effect of polymer compositions on drug release: As observed in the previous section, the sample ODF (40:60)and ODF(30:70)did not dissolve in the AS medium. Therefore, we did not continue with these samples for drug release studies. In order to investigate the effect of composition of Alg and Cas on the release rate, we selected the formulations ODF (70:30)48, and ODF (50:50)48. The release profile of Amx from these films in the artificial saliva of pH 6.4 is shown in Figure 10
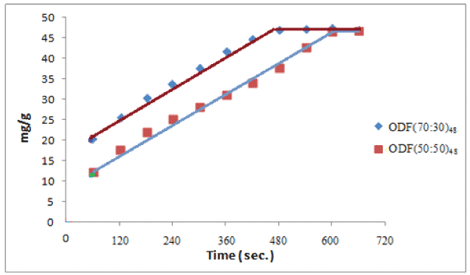
Figure 10: Dynamic release of drug Amx from the samples ODF (70:30)48, and ODF (50:50)48 in the PBS of pH 6.4
A close look at the release profiles reveals some interesting facts. The sample ODF (70:30)48 shows faster release and all the entrapped drug is released in about 480 s, a time quite close to the dissolution time of the film sample. On the other hand, the sample ODF (50:50)48 demonstrates a relatively slower release. The reason is that the sample contains greater Casein content and therefore not only it shows slower dissolution but it also has higher disintegration time. These two factors, namely enhanced dissolution and disintegration periods cause a slower release. Moreover, the additional physical cross-links by casein molecules could also be responsible for slower release. It is also noticeable that the two films exhibit ‘zero order profiles as discussed above. We also determined first order rata constants using the linear portion of the plots. The values of k0 for the samples ODF(70:30)48 and ODF(50:50)48 were found to be 3.498 and 3.541 mg/g/s respectively.
Sweeteners are the important part of the food products and are also used in pharmaceutical products intended to be disintegrated or dissolved in the oral cavity. Natural sweeteners as well as artificial sweeteners are used to improve the taste of the mouth dissolving formulations. The classical source of sweetener is sucrose (derived from cane or beet in the form of liquid or dry state), liquid glucose, dextrose, fructose, glucose and maltose. In order to investigate the effect of addition of sucrose on the disintegration/dissolution time, sample ODF(70:30) was selected. I prepared three samples, containing 50, 100 and 150 mg of sucrose per g of the film. The results are shown in Table 4. It can be seen that as the amount of sucrose in the film increases, disintegration/dissolution time also increases. It is attributable to the presence of additional interactions present within the films due to sucrose. It may be that sucrose molecules form H-bonding with polar groups of Alg and Casein.
| Amount (mg) | Sweetener (sucrose) | Saliva stimulating (malic acid) | Surfactant (sodium lauryl sulphate) |
| Disintegration time (sec)/ Dissolution Time (sec) | |||
| 50 | 60/460 | 55/465 | 45/450 |
| 100 | 65/470 | 70/475 | 50/465 |
| 150 | 67/470 | 70/480 | 60/470 |
Table 4: Effect of sweetener, saliva stimulating agent and Surfactant on the disintegration dissolution of films
Saliva stimulating agents are used to increase the rate of production of saliva, as it activates the salivary gland, which would aid in the faster disintegration of films. Mostly acids are used as salivary stimulants. Citric acid, malic acid, lactic acid, ascorbic acid and tartaric acid are the some examples of salivary stimulants [34]. Almost similar results were also obtained for the addition of malic acid as saliva stimulator and sodium lauryl sulphate as surfactant.
It may be concluded from the above study that sodium alginate/casein blend may be used as orally dissolving film (ODF) for the fast release of amoxicillin via oral mucosa route. The films take 5 to 8 min for complete dissolution in artificial saliva medium. Addition of sweetener, saliva stimulator and surfactant delay the dissolution process.
The authors report no declarations of interest.
Download Provisional PDF Here
Article Type: Research Article
Citation: Bajpai SK, Shah FF, Bajpai M, Jadaun M, Jyotishi P (2017) Dynamic release of Amoxicillin from Orally Dissolving Film (ODF) composed of Casein and Sodium alginate. J Drug Res Dev 3(3): doi http://dx.doi.org/10.16966/2470-1009.134
Copyright: © 2017 Bajpai SK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access