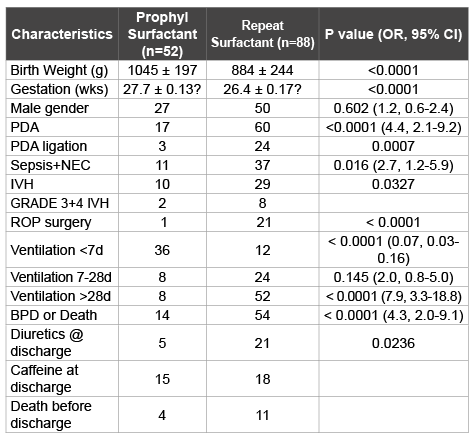
Table 1: Comparison of clinical characteristics between prophylactic
surfactant* and repeat surfactant groups
*Beractant

Bhatt-Mehta V1,2* Schmacher RE2 Dechert RE2 Sarkar S2
1College of Pharmacy, University of Michigan, USA*Corresponding author: Bhatt-Mehta V, Pharm.D, MS, FCCP, Clinical Professor, Pharmacy, Pediatrics and Communicable Diseases, University of Michigan, USA, Tel: (734)-936-8985; E-mail: varsham@umich.edu
Objective: We determined if restricting repeat doses of beractant, by using high-threshold criteria for respiratory support, increased the risk of the composite primary outcome of BPD or death before hospital discharge.
Methods: A total of 140 infants of <29 weeks gestation who received prophylactic beractant soon after birth and admitted to a Neonatal Intensive Care Unit were reassessed 12 hours after the initial dose for retreatment if the infant remained intubated and required at least 40% inspired oxygen with a MAP >10 cm H2O, and compliance of <0.5 ml/cm H2 O.
Multivariate analysis identified which risk factors from a set of a priori predictors including the need for Survanta retreatment could predict the primary outcome.
Results: Eighty-eight (59%) of the 140 infants reached the retreatment criteria and received repeat doses of Survanta. Sixty-eight (49%) infants developed BPD or died. Infants who developed BPD or died were younger and smaller; were more likely to have PDA, NEC or sepsis, longer (>28 days) stay on mechanical ventilation, and receive retreatment with Survanta. On forward stepwise logistic regression analysis of a priori risk factors only the need of ventilation >28d (p<0.001, OR 7.3, 95% CI 2.7-19.5) was independently associated with increased risk of primary outcome.
Conclusion: Restricting repeat doses of Survanta did not increase the risk of development of BPD or death in preterm infants with RDS.
Surfactant replacement therapy in the treatment of neonatal respiratory distress syndrome secondary to immaturity-related surfactant deficiency has been established as an effective and safe therapy since the early 1990s [1]. Many systematic reviews of randomized controlled trials have confirmed that surfactant replacement reduces initial inspired oxygen and ventilation requirements during the first weeks of life in premature neonates as well as the incidence of respiratory distress syndrome, death, pneumothorax, and pulmonary interstitial emphysema [2-5]. Prophylactic or preventive surfactant administration is defined as intubation and surfactant administration to infants at high risk of developing neonatal respiratory distress syndrome (RDS) prior to symptoms of RDS occurring. In many clinical trials this strategy was implemented as surfactant administration before the onset of respiratory symptoms or efforts, before initial resuscitation efforts, or, most commonly, after initial resuscitation but within 10 to 30 minutes after birth [1]. This contrasts with a rescue or treatment surfactant strategy, in which surfactant is given to preterm infants with a diagnosis of RDS based on signs and symptoms and chest radiographic findings consistent with RDS. Preterm infants born at or earlier than 30 weeks’ gestation have benefited from both prophylactic and rescue surfactant administration [4,6]. However, infants receiving prophylactic surfactant have had a lower incidence and severity of respiratory distress compared with those treated with rescue surfactant. Infants receiving prophylactic surfactant also have encountered fewer complications of RDS, such as death, pneumothorax, pulmonary interstitial emphysema, and the combined outcome of bronchopulmonary dysplasia or death [7-14]. Although limited, the results of studies on surfactant treatment of RDS indicate that surfactant administered prophylactically or as soon as possible in the course of respiratory distress is more effective than late rescue surfactant at improving outcomes [15]. Administered surfactant is phagocotized, repackaged, and resecreted by the lungs. Anecdotal experience and controlled trials have found that some amount of retreatment is still beneficial in selected cases. However, surfactant administration protocols used during development of beractant and colfesceril palmitate required minimal levels of respiratory support for retreatment. Current manufacturer recommendations for beractant suggest redosing no sooner than 6 h after the preceding dose if the infant remains intubated and requires at least 30% inspired oxygen [2- 4]. Administration of beractant every 12 hours vs every 6 hours as recommended by the manufacturer found no difference in duration of mechanical ventilation and length of hospital stay [16]. Based on this introduction it is evident that there is still significant debate regarding the need for the initial and subsequent surfactant treatment and the frequency of administration for improvement of associated morbidity and mortality due to neonatal RDS. This study was designed to test the hypothesis that restricting subsequent doses of surfactant after the prophylactic dose may alter the risk for development of BPD or death. Reducing the number of unnecessary repeat doses would represent a significant cost-saving and decrease complications associated with surfactant administration. Accordingly, we introduced a practice change in early 2008 whereby prophylactic surfactant administration was limited to infants <28 weeks gestation and repeat surfactant doses were administered based on strict criteria which included assessment for need of repeat dose at 12 hours from the initial prophylactic dose (vs 6 hours recommended by the manufacturer of beractant, our formulary surfactant of choice) and the need for a compliance of <0.5 ml/cm of H2 O or inspired oxygen of greater than 40% to qualify for repeat dose(s).
The protocol provided very specific guidelines for ventilator management and qualification for repeat (rescue) dose(s). Briefly, surfactant redosing (subsequent to initial prophylactic dose) was to be considered if the infant still remained intubated 12 hours after the prophylactic dose of surfactant and if FiO2 >0.4, or mean airway pressure >10 cm H2 O, or Cdyn <0.5. One to two additional doses were allowed. Attending approval was required to give more than two doses. In this protocol the gestational age for prophylactic administration was <28 weeks and caffeine was introduced for routine prophylaxis against BPD starting on day of life one. The objective of this study was to determine if restricting administration of repeat doses of beractant by using highthreshold criteria for respiratory support incorporated in the treatment protocol increased the risk of the composite outcome of BPD or death before hospital discharge.
This was a single-center retrospective cohort study of premature neonates (≤ 28 weeks GA) receiving surfactant prophylaxis and caffeine according to a pre-existing protocol in the Brandon NICU at the University of Michigan (UM) between January 2010 and November 2012. This study received waiver of consent from the University of Michigan Investigational Review Board. A total of 140 preterm infants of ≤ 28 weeks gestation who survived at least until 4 weeks of age, had received prophylactic berectant soon after birth (within 30 minutes), and were reassessed 12 hours after the initial dose for retreatment were included in the study. Retreatment with beractant was administered if the infant remained intubated and required FiO2 >0.4 with a MAP>10 cm H2 O, or Compliance of <0.5 ml/ cm H2 O. Infants who met the study criteria were identified from the Vermont Oxford Network (VON) database at University of Michigan. Data collection included neonatal and maternal demographics, maternal medication use, initial respiratory status, length of stay (LOS), and total LOS in the NICU, duration of respiratory support, surfactant use, steroid use, caffeine use, and other relevant medications. The number of doses of surfactant from 0 to 96 hours after birth, diuretic, and caffeine use during hospitalization was also recorded. BPD was defined according to VON criteria as the need for oxygen at 36 weeks corrected gestational age. All other definitions for the clinical characteristics and the risk factors of BPD or death were also according to VON criteria. Appropriate inferential and description statistical tests were used to analyze the data. Forward stepwise logistic regression analyses identified which risk factors from a set of a priori predictors could predict the primary outcome. The selected risk factors included gestation, birth weight, gender, PDA, sepsis or NEC, the need for ventilator support for <7 days, 7-28 days, or >28 days, and the need for berectant retreatment. These a priori risk factors except the need for berectant retreatment have been shown to be predictors of the composite outcome of BPD or death in previous studies [17]. P-value of ≤ 0.05 was considered significant.
A total of 88 (59%) of 140 infants reached the retreatment criteria and received repeat doses of beractant. Sixty-eight (49%) infants developed BPD or died before discharge from hospital. Comparison of clinical characteristics between prophylactic beractant and repeat beractant groups appear in Table 1. Infants in the prophylactic group were of higher gestation and birth weight. Preterm infants who qualified for repeat doses of surfactant were in general sicker and had an incidence of sepsis and/ or NEC, and patent ductus arteriosus (PDA). Fewer neonates in the prophylactic surfactant group required mechanical ventilation greater than 28 days compared to repeat surfactant group and had a lower incidence of BPD or death compared with the repeat surfactant group. Univariate analysis (Table 2) of these risk factors confirmed gestational age, birth weight, presence of sepsis and/or necrotizing enterocolitis (NEC), need for mechanical ventilation at >28 days postnatal age and need for repeat surfactant as significant predictors of the composite outcome. However, in multivariate regression analysis (Table 3), only need for mechanical ventilation at >28 days emerged as a significant risk factor for BPD or death, this composite outcome was not necessarily related to early intervention in the form of survanta retreatment.

Table 1: Comparison of clinical characteristics between prophylactic
surfactant* and repeat surfactant groups
*Beractant
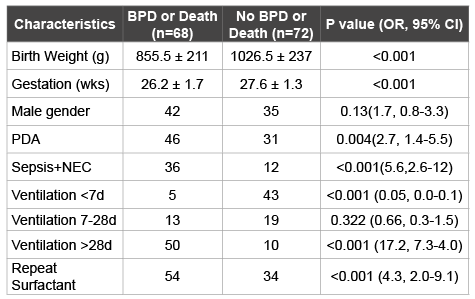
Table 2: Univariate analysis of a set of a priori risk factors for prediction of BPD or death before discharge

Table 3: Multivariate analysis of the risk factors for prediction of BPD or death before discharge
Surfactant replacement is associated with an absolute increase in the number of preterm infants who survive with and without disabilities [18-21]. Studies of either continuous positive airway pressure alone or exogenous prophylactic surfactants and rapid extubation to continuous positive airway pressure have suggested that the need for surfactant replacement and incidence of bronchopulmonary dysplasia in extremely preterm infants may be reduced [22-27]. There is a general consensus that severity of both RDS and BPD has decreased (sparse data) and that the major variable driving BPD is birth weight and gestational age. Surfactant treatments increase survival but probably do not decrease BPD. Randomized controlled trials including the Coin Trial [26] suggest that CPAP is comparable to intubation +/- surfactant for initial stabilization of infants in the delivery room. In the COIN trial, CPAP did not significantly reduce the incidence of death or BPD compared with intubation. The Coin Trial/ Support Trial showed that perhaps 50% of ELBW infants do not have RDS at birth severe enough to require surfactant treatment [26,27]. It appears that death or BPD is outcomes with casualties not necessarily related to the early interventions performed immediately after birth.
Our study, performed under strict criteria for subsequent surfactant administration following initial prophylactic dose, showed that restricted use of repeat doses of surfactant after the 164 prophylactic doses was not independently associated with the risk for development of BPD or 165 deaths. In our cohort the ELBW infants who qualified for repeat doses of surfactant (despite strict 166 criteria) were smaller in size and sicker with higher incidence of patent ductus arteriosus and necrotizing enterocolitis. They also were on mechanical ventilation longer than the prophylactic group and had a much higher incidence of BPD or death. In the final analysis the number of days on mechanical ventilation seemed to be associated significantly with the outcome of 170 interests. Populations for the predictive models of BPD risk following prophylactic or rescue surfactant treatment in previous studies were from the presurfactant era or before widespread use of prenatal steroids. In these studies BPD was defined as need for oxygen at postnatal age 28 days. Death was not generally included as a competing outcome. These studies also did not typically include postnatal age and therefore could not quantify the variable contribution of neonatal exposures over time. Our patient population is more recent following almost universal use of antenatal steroids as well as inclusion of a composite outcome of BPD or death. We chose to define BPD as need for oxygen at 36 weeks corrected gestational age based on the Vermont Oxford Network database definition. This definition allows for all infants needing supplemental oxygen by any method at this CGA allowing for wider applicability of our results.
Limitations of the study include its single center, retrospective study design, and exclusion of infants who died before day of life 28. We also did not use the NICHD physiological definition of BPD. We thus conclude that prolonged mechanical ventilation >28 days and not the need for surfactant retreatment is associated with risk for development of BPD or death before discharge. BPD or deaths are outcomes with casualties not related to early interventions for respiratory distress in the newborn.
In conclusion, restricted use of repeat doses of surfactant after the prophylactic dose was not independently associated with the risk for development of BPD or death. We speculate that reducing the number of unnecessary subsequent doses of surfactant would result in a substantial cost-saving from less utilization of the drug and reduce the risk of adverse effects associated with surfactant treatment. Innovation to improve noninvasive ventilation strategies will probably play a bigger role in improving the outcome of BPD or death in ELBW infants.
The authors acknowledge the contribution of the following students to this research.
KS, TH, RD
The authors declare no conflict of interest.
Download Provisional PDF Here
Article Type: Research Article
Citation: Bhatt-Mehta V, Schmacher RE, Dechert RE, Sarkar S (2016) Does Restricted Use of Repeat Doses of Surfactant after the Prophylactic Dose Increase the Risk of BPD or Death in Preterm Infants? J Drug Res Dev 2(3): doi http://dx.doi. org/10.16966/2470-1009.119
Copyright: © Bhatt-Mehta V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access