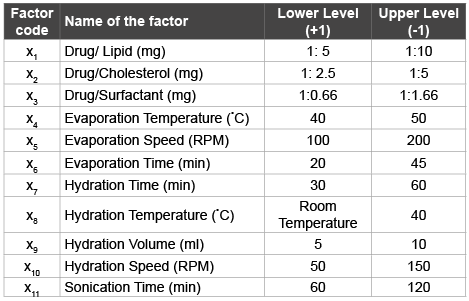
Table 1: Low and high levels of the eleven examined factors

Vanaja K1,2* Shobha Rani RH1 Narendra C2
1Department of Pharmaceutics, Al-Ameen College of Pharmacy, Hosur road, Bangalore, India*Corresponding author: Vanaja K, Assistant Professor, Visveswarapura Institute of Pharmaceutical Sciences, BSK II stage, Bangalore, India, E-mail: vanaja_ceutics@yahoo.com
Methotrexate (MTX) being the widely used biologic agent is used to treat severe and/or disabling psoriasis but precipitates various side effects on oral therapy. Encapsulating methotrexate in flexible liposomes for delivery through skin could lead to better therapeutic action and reduce side effects. In order to screen out the various factors during liposome formulation development, Plackett Burman screening design was applied. Three formulation variables and eight process variables were identified and one factor plot was used to predict the response. The present study was aimed at identifying the important factors among the various factors, which could attribute to obtain highest drug entrapment during formulation of flexible liposomes. Results obtained proved that application of a screening design such as Plackett-Burman design efficiently identified the most significant factors. Identified significant factors were further optimized using Central Composite Design using the software Design Expert v 6.0. Three optimal solutions suggested by the design were characterized for entrapment efficiency, particle size, zeta potential and microscopical analysis. Results demonstrate the feasibility of applying statistical designs in the development of flexible liposomes of methotrexate.
Psoriasis is a chronic skin disorder affecting 0.1% to 3% of the world population. Methotrexate (MTX) is the most widely used biologic agent in the treatment of psoriasis [1]. MTX has been used to treat severe and/ or disabling psoriasis but oral therapy precipitates various side effects [2]. In order to overcome these side effects, one approach is to administer MTX topically. A topical drug product is designed to deliver drug into the skin for treating dermal disorders with skin being the target site [3]. But skin is a barrier to the absorption of topically administered drugs. Although the incidence of systemic toxicity in topical therapy is far less than encountered with systemic routes of drug delivery, topical therapy may fall short in delivering a clinically sufficient local concentration of drug. This necessitates a need for new drug carrier systems for treating skin disorders. One of the possibilities for increasing skin permeation of drugs is the use of liposomal systems. In the last few decades, liposome encapsulated drug delivery via the skin has undergone intense investigations. Classic or the traditional liposomes are of little value as carriers for transdermal drug delivery because they do not deeply penetrate the skin. Only specially designed vesicles have shown to be capable of allowing such enhancement [4]. Novel types of liposomes known as flexible liposomes have been designed with improved bilayer composition and preparation process to push liposomes more deeply into the skin. The basic rationale of such liposome design is that all carriers contain atleast one highly polar amphiphilic components such as sodium cholate, sodium deoxycholate or surfactants of pharmaceutical quality. The provisos have high membrane elasticity, permeability and vesicle stability confirming the flexible liposome mediated drug delivery into the skin [5].
In order to support and justify flexible regulatory paths for innovation in manufacturing and post approval changes, a scientific understanding of the relevant multi-factorial relationships and means to evaluate the applicability of this knowledge in different scenarios is required. This is achieved through use of multivariate mathematical approaches, such as statistical design of experiments (DOE). Applying DOE throughout a pharmaceutical process is the key to quality as it maximizes the information content of a small number of experiments. Pharmaceutical processes are complex systems affected by many factors. DOE identifies optimal formulation and process conditions for these systems and provides understanding of the underlying relationships.
During liposome formulation development, the formulator would be interested in determining which process or formulation variables affect the response the most. In order to estimate the effects of factors, various screening designs are available that can aid the formulator’s choice of formulation components which will optimize one or more product attributes. Plackett and Burman (PB) devised orthogonal arrays are useful for screening that yield unbiased estimates of all main effects in the smallest design possible. Various number or ‘n’ factors can be screened in a ‘n + 1’ run PB design. The projective property of PB design is that it allows the experimenter to follow up an initial design with runs that allows an efficient separation of main effects and interaction effects [6-9].
As PB designs are Resolution III designs with the attribute of requiring fewest numbers of runs, but do not allow the estimation of interactions between factors, it can only identify the significant main factors that make up the possible significant interactions. Further the important main factors can be analyzed using a rotatable central composite Box-Wilson design. The central composite design consists of a ‘cube’ portion made up of the design points from a 2K factorial or 2K-1 fraction factorial design, star points and center points [10].
Thus the present study aim at identifying the important factors using a screening design such as Plackett Burman design and optimize the formulation using Central Composite.
Methotrexate was obtained from Biochem Pharmaceuticals, Mumbai, India. Soy Phosphotidyl Choline, Cholesterol, Sodium Cholate were obtained from Himedia Laboratories, Mumbai, India. Propylene glycol and glycerine were purchased from SRS scientific, Bangalore, India. Carbopol P 940 was a gift sample from Karnataka Antibiotics Private Limited, India. Chloroform and methanol were obtained from Qualigens, India. Double distilled water (Millipore) was used throughout.
Briefly, lipid phase comprised soya phosphotidylcholine (SPC), cholesterol and surfactant (sodium cholate) dissolved in CHCl3 : MeOH (2:1 v/v) mixture in a dry round bottom flask. Organic solvents were removed by a vacuum evaporator (Rotavapor-R, W.Büchi, Flawil Schweiz) above the lipid transition temperature to obtain a uniform, thin lipid film on the wall of the flask. The deposited lipid film was hydrated with phosphate buffered saline (pH 7.4) containing methotrexate, for 60 min at room temperature with continuous rotation for 1 hr. Small unilamellar (SUV) liposomes were obtained by subjecting the dispersions to probe sonication (Ultrosonic Processor, UP200S, Hielscher Ultrasound Technology) for 2 to 6 mins using cooling pads. Finally the liposomal dispersions prepared were stored at room temperature for 2 hrs to anneal any structural defects.
Methotrexate concentration was quantified by HPLC using the method reported earlier with slight modifications, at 302 nm using Shimadzu LV AT-10VP liquid chromatography equipped with a variable UV detector and a computer integrating apparatus [11]. The column used was Phenomenex Luna 5u C18 (2) 100 A, 250 x 4.60 mm. The mobile phase consisted of a mixture of acetonitrile and phosphate citrate buffer (90:10) pH 6 at a flow rate of 1ml/min at ambient temperature with retention time (R.T.) of 7.2 ± 0.3 min.
From the mentioned procedure of thin film hydration, it is evident that the design of an efficient liposomal preparation is a multivariate procedure in which many factors could affect the desired liposomal properties. It is difficult for a formulator to guess the effects of these factors on the desired response.
Eleven variables with the potential to influence drug entrapment were examined. Formulation variables studied were drug/lipid, drug/ cholesterol and drug/surfactant. Process variables examined were evaporation temperature, evaporation speed, evaporation time, hydration time, hydration temperature, hydration volume, hydration speed and sonication time. A Plackett-Burman (PB) design with 12 experiments including three replicates was constructed using the software Design expert V. 6.05. Using this design the magnitude of effect of each variable on the resulting response can be estimated independently of all other tested variables. Each variable was tested at two levels low (-1) and high level (+1) and the measured response was percent drug entrapment (PDE). Ratio of drug/lipid was varied from 1:5 mg to 1:10 mg at low and high level respectively, drug/cholesterol were 1:2.5 mg to 1:0.5 (mg) at low and high level respectively, whereas drug/surfactant was varied from 1:0.66 (mg) to 1:1.66 mg at low and high level respectively. Other process variables were varied at low and high level (Table 1). As per the PB experimental design, flexible liposomes were prepared using thin film hydration. Entrapment efficiency was determined as described earlier.

Table 1: Low and high levels of the eleven examined factors
A randomized rotatable CCD was generated using the software- Design Expert V. 6.0.5 © Stat ease Inc., U.S.A. According to the model it contains four full factorial design points, four axial points and three center points varied at two levels. The design involved the estimation of two significant factors identified from PBSD at high and low level (Table 3). Higher and lower levels of each factor were coded as +1 and -1 respectively and the mean value as 0. Addition of center points allow us to detect non-linearity in the responses, hence were repeated three times to get an estimate of experimental or pure error. Axial points were also included which are levels of the factors under investigation, located at a distance outside the original high or low factor range [10,12]. The CCD contained five levels of each factor: low axial, low factorial, center, high axial and high factorial. The critical response variable measured was drug entrapment. Actual fitting of the model was computed using statistical software. Run order was randomized to ensure against the effects of time related variables and also to satisfy the statistical requirement of independence of observations. Coded CCD matrix and results are presented in (Table 4).
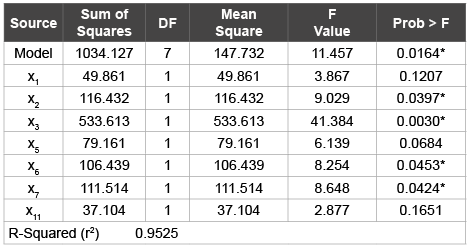
Table 2: Analysis of Variance (ANOVA) obtained from Plackett Burman
screening design
*(P< 0.05)
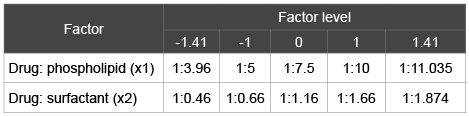
Table 3: Factors and their corresponding levels for the construction of CCD
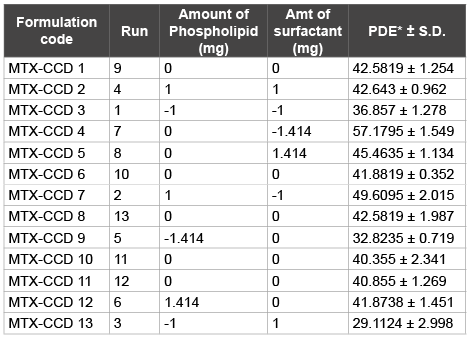
Table 4: Coded Matrix of central composite design and entrapment efficiency
* Mean of three trials
PDE: percent drug entrapment and S.D. standard deviation
Check point experiments was performed to confirm the utility of obtained 3D diagrams and polynomial equation in the formulation of MTX liposomes using thin film hydration. Values of independent variables (X1 and X2 ) were taken from three checkpoints suggested by the software. Liposomes were prepared experimentally by the taking the amounts of independent variables (X1 and X2 ) and mean PDE was determined. The predicted PDE was compared with the observed PDE (Table 5) Further the optimal solutions were characterized for particle size, polydispersity index, zeta potential, in-vitro release and microscopic analysis.
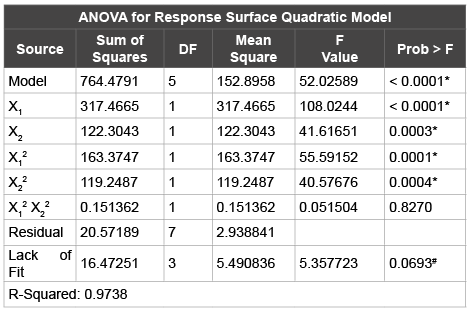
Table 5: : Analysis of variance (ANOVA) results of response surface
quadratic model using sodium cholate as the surfactant/edge activator
*(P< 0.05)
# (P > 0.05)
d.f. = degrees of freedom
F = Measurement of distance between individual distributions
Probability ‘P’ less than 0.05 indicate model terms are significant
Percentage entrapment efficiency (% EE) was determined after separation of free and entrapped drug. 5 ml of liposome suspension was subjected to ultracentrifugation (Beckman L7 ultracentrifuge, Beckmann Coulter GmbH) for 50 mins at 40,000 RPM at 6 degreesC. Methotrexate content was determined indirectly from the free concentration in the supernatant after ultracentrifugation using HPLC.
$$\% EE\, = {{{\rm{Amount}}\,{\rm{of }}\,{\rm{drug}}\,{\rm{entrapped}}} \over {{\rm{Total}}\,{\rm{amount}}\,{\rm{of}}\,{\rm{drug}}\,}}\, \times 100$$
Particle size (Z-average mean size and polydispersity index) and ζ - potential were determined by dynamic light scattering (DLS) and Laser Doppler Electrophoresis (LDE), respectively, using a Nanosizer (Nano-ZS, Nanoseries, Malvern Instruments, UK) after appropriate dilution of liposome preparation. Six independent measurements were performed in each case. Mass distribution of particle size with polydispersity index as well as the electrophoretic mobility was obtained. Sizes are reported as the Z-average mean diameter for the hydrodynamic diameter (nm) along with polydispersity index. Zeta potential (mV) was calculated from electrophoretic mobility in the electric field [13-17].
In vitro study was carried out using Franz diffusion cell across human cadaver skin at 35 ± 2°C. The receptor phase (PBS pH 7.4) was constantly stirred by magnetic stirrer at 100 rpm. The effective permeation area of the diffusion cell and receptor cell volume was 2.5 cm2 and 75 ml, respectively. The deformable liposomal MTX gel formulations (dispersed in varying concentrations of carbopol gel base) containing the final concentration of 0.25% w/w of MTX was used for the in vitro release studies [18,19]. Liposomal MTX gel containing MTX equivalent to 2.5mg was applied to the surface of the cadaver skin. Samples were withdrawn through the sampling port of the diffusion cell at predetermined time intervals over 24 hr and analyzed using HPLC at 302 nm.
The irritancy of MTX liposomal gel; 0.25% w/w MTX was determined in male Wistar rats (150-200 gms). The animals were housed in an airconditioned room (20° C) and hair of the back was trimmed short, 24 hr before the beginning of the test. The animals were divided into two group of six each. First group of rat received optimized liposomal formulation and second group received the marketed formulation. Two squares were drawn on each side of the back of each rat, and 0.5 gms of each formulation were applied on each square. The treated animal was protected by using nylon mesh, supported by the plastic squares having small pores, kept above the treated area. After exposure for 72 hr, the test substance was removed and exposed skin was scored for the formation of edema (graded 0–4) and erythema (graded 0–4) as per Draize Evaluation of Dermal Reactions (Ethical clearance was obtained from Institutional ethical committee, Al-Ameen College of Pharmacy, approved by CPCSEA) [20].
To add to the “therapeutic ladder” of psoriasis treatment, ultradeformable liposomes of methotrexate (MTX) were designed and formulated with biocompatible ingredients such as lipid, sodium cholate and cholesterol. It was observed that higher amount of lipid and lower amount of sodium cholate resulted in higher percent drug entrapment (PDE). The possible explanation could be due to the fact that MTX being a polar molecule gets encapsulated into the aqueous core requiring higher amount of lipid to form the lipid lamellae. As reported, incorporation of the right proportion of membrane softer (surfactant) plays an important role in ultradeformable liposomes [21]. Higher amount of surfactant leads to aggregate disintegration, typically in form of amphiphats, reducing the entrapment efficiency of the drug.
This study was undertaken with an aim of identifying those variables using PBSD, which can be controlled in order to formulate flexible liposomes with maximum drug entrapment.
The target PDE was fixed at maximum entrapment. After collection of data from 12 runs and response calculated, the system was ready for analysis beginning with the calculation of regression coefficient. PBD are based on Hadamard matrices and the results are interpreted using multiple linear equation121
y = a0 + a1 x1 + a2 x2 + ...........+ a11x11
Where ‘y’ stands for the predicted response, x1 - x 11 stands for the settings (factors), a1– a11 are the respective coefficients and a0 stands for the intercept of mean.
The multiple linear equations giving the mathematical relationship between each factor was found to be
Percent drug entrapment = +29.55418
+ 0.81537 x Drug/Lipid
+12.45967 x Lipid/Cholesterol
-17.78244 x Lipid/surfactant
-0.051368 x Evaporation Speed
-0.23826 x Evaporation Time
+0.20323 x Hydration Time
-0.14067 x Sonication Time
After the estimation of the factor regression co-efficient, the significant factors affecting the dependent variable (PDE) was determined by performing Analysis of Variance (ANOVA). Statistical analysis of the results showing the estimated effects is presented in (Table 2). ANOVA presents the sum of squares (SS) which is used to estimate the main effects of the factor, the F-ratios (F) as the ratio of the respective mean-square effect (MS) and the mean-square error. ‘P’-probability values indicate the significant factors affecting the response74,75. The model was significant with ‘P’ value 0.0164 (P <0.05). The ‘P’ values in (table 2) identify the main effect of each factor to be statistically significant (P < 0.05) or marginally significant (P < 0.10). The factors with the asterisks viz, x1 : drug/lipid, x2 : drug/surfactant, x6 : evaporation time and x7 : hydration time (p < 0.05) significantly affected the response. There was good correlation between the observed and fitted values (R2 = 0.982).
With the application of Plackett Burman screening design, we could identify the significant variables affecting PDE. This considerably reduced the number of factors (from eleven factors to four factors) to be investigated in further studies.
In order to optimize the formulation variables and study the interaction between the main effects, response surface methodology (specifically; randomized central composite designs) was applied.
Thirteen batches of UDL’s of MTX were prepared by varying two independent variables and their levels were selected based on PBSD (Figure 1). Lipid (x1 ) and sodium cholate (x2 ) were the independent variables and PDE (dependent variable) of the 13 batches (prepared in triplicates) was determined and the results recorded in (Table 4).
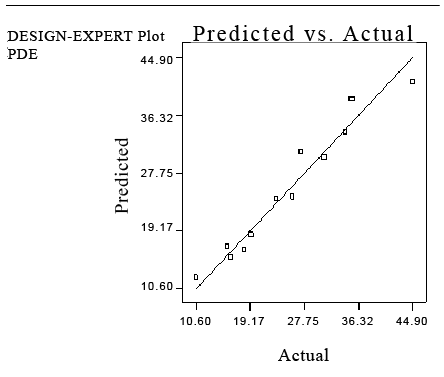
Figure 1: Correlation between actual and predicted values of percent drug entrapment (PDE) with PBSD.
A substantial increase in percent drug entrapment achieved in liposomes was between 29.1124 ± 2.998 and 57.1795 ± 1.549. At +1 level of x1 (lipid) and -1 level of x2 (sodium cholate), the PDE ± was 49.6095 ± 2.015, whereas -1 level of x1 (lipid) and +1 level of x2 (sodium cholate) the PDE ± was 29.1124 ± 2.998.
The polynomial equation of the quadratic model giving the mathematical relationship between each factor was
PDE = 12.164
+ 0.9312 *lipid
- 3.1753*sodium cholate
- 0.0034* (lipid) 2
+ 0.0736 * (sodium cholate)2
+ 0.000692 *lipid x sodium cholate
Neglecting nonsignificant (P>0.05) terms from the full model established a reduced model
PDE = 12.164
+ 0.9312 *lipid
- 3.1753*sodium cholate
- 0.0034* (lipid) 2
+ 0.0736 * (sodium cholate)2
After the estimation of the factor regression co-efficient, the significant factors affecting the dependent variable (PDE) was determined by performing Analysis of Variance (ANOVA). Statistical analysis of the results showing the estimated effects is presented in (Table 5). ANOVA presents the sum of squares (SS) which is used to estimate the main effects of the factor, the F-ratios (F) as the ratio of the respective mean-square effect (MS) and the mean-square error. ‘P’-probability values indicate the significant factors affecting the response. ANOVA indicated that the PDE is best fit to a quadratic model rather than a linear model. The model F-value was 52.03 with probability ‘P’ value of <0.0001 which implies that there is only a 0.01 % chance that the model F-value could have occurred due to noise. The predicted R-squared of 0.8426 was in reasonable agreement with the Adjusted R-square of 0.9551 indicating the fitting of the quadratic model. Lack of fit was good with non-significant ‘P’ value of 0.0693.
The mathematical model indicated that the quadratic terms x1 , x2 , (x1 )2 , (x2 )2 were found to be significantly (p<0.05) affecting PDE. Hence lipid and sodium cholate played an important role in PDE of MTX.
The combined effect of factor x1 and x2 can be further elucidated with the help of response surface plot and perturbation plot (Figures 2 and 3), which indicate a positive effect of lipid concentration (x1 ) i.e., increase in concentration of lipid increased percent MTX entrapment whereas a negative effect was observed in case of sodium cholate concentration (x2 ) i.e., increase in surfactant concentration decreased PDE. Figure 4 represents the actual response values compared to that of predicted values.
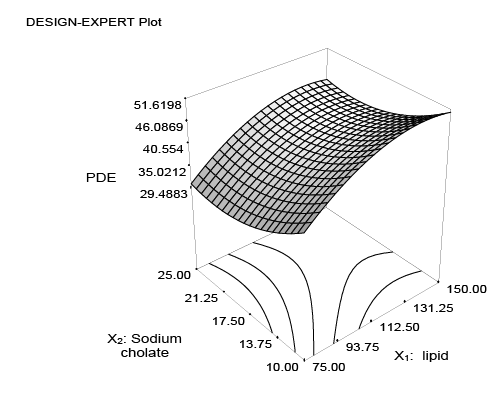
Figure 2: 3-D response surface plot showing the effect of lipid (x1 ) and surfactant (x2 ) on the response (percent drug entrapment) of UDL containing sodium cholate
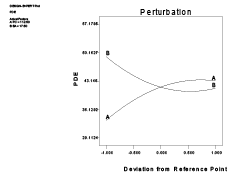
Figure 3: Perturbation plot showing the effect of x1 (lipid) and x2 (surfactant) on percent drug entrapment (PDE) of UDL containing sodium cholate
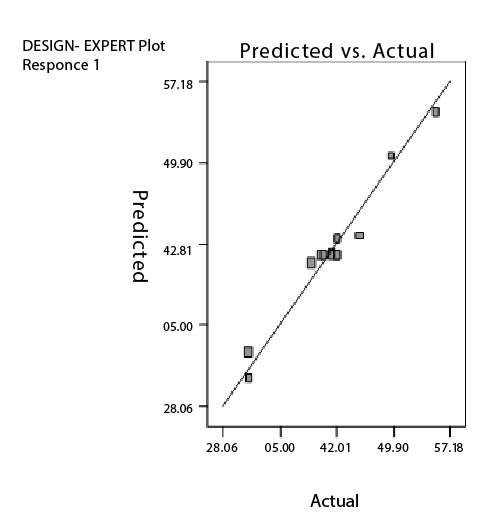
Figure 4: Correlation between actual and predicted values of Percent drug entrapment (PDE) of UDL containing sodium cholate
Regression analysis, ANOVA and diagnostic plots revealed that +1 level of lipid and -1 level of sodium cholate gave higher PDE. The possible explanation could be due to the fact that MTX being a polar molecule gets encapsulated into the aqueous core requiring higher amount of lipid to form the lipid lamellae. As mentioned earlier, incorporation of the right proportion of membrane softer (surfactant) plays an important role in ultradeformable liposomes. Higher amount of surfactant leads to aggregate disintegration, typically in form of amphiphats, reducing the entrapment efficiency of the drug. As these UDL’s are aimed to be applied topically, higher surfactant concentration may also affect the psoriatic skin. All these results are in accordance with the literature available [3,5,6,8,21]. Thus in order to obtain an optimized setting of lipid and sodium cholate, numerical optimization by the desirability approach was used. The process was optimized for the dependent variable (response) and optimized formulations were arrived at maximizing the lipid concentration and minimizing the sodium cholate to obtain higher PDE. Three numerically optimized solutions with the constraints of lipid & sodium cholate concentration in range and PDE in target of 50% were obtained. To gainsay the reliability of the response surface model, they were prepared according the predicted model and evaluated for PDE. Results in table 6, showed a good relationship between the experiment and predicted values, which confirms the practicability and validity of the model.
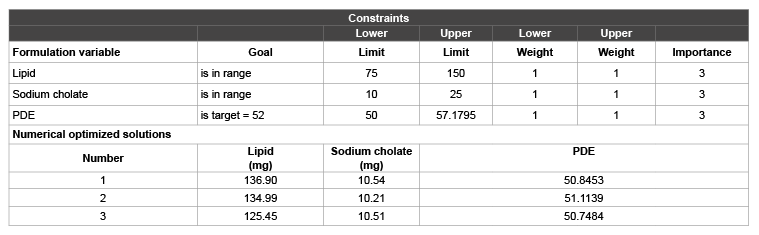
Table 6: Constraints & Numerical optimization solutions of ultra deformable liposomes of MTX with sodium cholate as surfactant/edge activator (Design expert V. 6.05, Stat Ease, Inc., USA)
A check point experiment was performed to confirm the utility of design. Three optimal solutions as suggested by the software were further formulated using thin film hydration and examined for PDE. Observed response (PDE) was similar to the response predicted by Design Expert software with percentage deviation less than 5%.
The Z-average size (nm) corresponding to the hydrodynamic radius was determined using Malvernizer. All the vesicles formed were unilamellar with Z-average < 120 nm. The Z-average size (n =3) of MTXSC1, MTX-SC2, MTX-SC3 was 112.4, 101.3 and 97.00 nm respectively. The Polydispersity index (PI), which measures the level of homogeneity of particle sizes revealed that the particle size distribution was unimodal with PI (which is the width of the particle size distribution curve) of 0.160, 0.128, 0.125 for MTX-SC1, MTX-SC2, MTX-SC3 respectively. The mean zeta potential values of MTX liposomes containing sodium cholate as the surfactant were -18.84, -18.52 & -18.6 mV for MTX-SC 1, MTX-SC 2 & MTX-SC 3 respectively (Table 7).
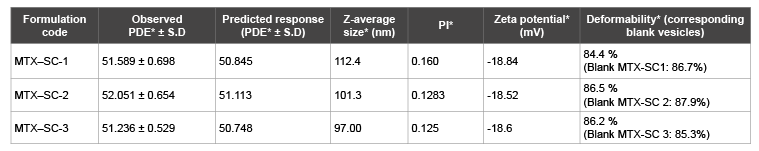
Table 7: Characterization of ultradeformable liposomes of MTX
*- Mean of three trials (n = 3); PDE ± S.D; Percent drug entrapment ± standard deviation; PI: Poly dispersity index (n= 3)
The deformability test of blank liposomes and MTX liposomes formulations was measured by comparing the volumes before and after extrusion through sheets of 50 nm polycarbonate membrane. Without MTX, the percentages of extruded volume for Blank SC – OS1, Blank SC – OS2, Blank SC – OS 3 were 86.7%, 87.9% and 85.3% respectively. Percentages of extruded volume of formulations with MTX i.e., MTXSC1, MTX-SC2, MTX-SC3 was 84.4%, 86.5% and 86.2% respectively. Microscopical examination revealed that the liposomes were unilamellar vesicles. In agreement with the light scattering size measurements, liposomes presented a size in the range of 95-115 nm.
In vitro permeation studies give us valuable information about the behavior of the delivery system in vivo. Physiological buffer, PBS (pH 7.4) was used as the receptor fluid in Franz diffusion cells to investigate the potential of ultra deformable liposomes to enhance drug transport. In order to examine the drug permeation pattern, in vitro permeation study was carried out using Franz diffusion cell across human cadaver skin. Permeation profile of liposomal MTX gel with different concentration of Carbopol is shown in (Figure 5). The values of transdermal flux for all the gel formulations was found to be between 33.582 mcg/cm2 /hr to 50.746 mcg/cm2 /hr with MTX – LG4 (1% Carbopol gel base) showed a controlled and sustained release of the drug.
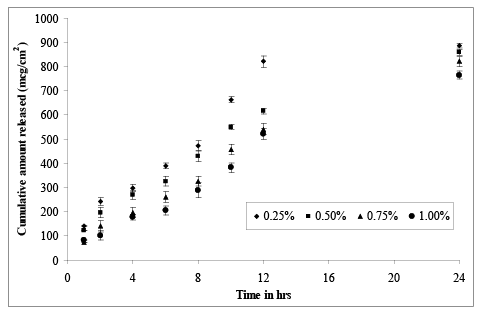
Figure 5: Permeation profile of liposomal MTX gel from different percentages of carbopol
After 24 and 72 hours of application of liposomal MTX gel and Plain MTX gel, the test sites were examined for dermal reactions in accordance with the Federal Hazardous Substance Act (FHSA)- recommended Draize scoring. The Primary Irritation Index (P.I.I.) of the test article was calculated following test completion. The Primary Irritation Index of the formulations was found to be 0.00; indicating the absence of irritation on the skin of the rats.
Under the conditions of this test, the formulations (liposomal MTX gel and plain MTX gel) were not primary skin irritants. The Primary Irritation Index was less than 5.00, which indicated the safety of both the formulations.
Application of a screening design such as Plackett-Burman design (PBD) as described reduced considerably the number of liposomal preparations and giving as much as possible information on the effects of the variables. PBD efficiently identified the most significant factors. Lipid and surfactant concentration significantly affected percent drug entrapment. An optimum formula to maximum PDE was derived from CCD in form a polynomial equation. Optimization the process variables and applying statistical designs led to increase in PDE to 57%. Among the various factors governing the delivery of drugs into the skin from topically applied formulations, particle size, and charge on the liposomal surface influence drug deposition into the deeper layers of skin.
All the formulations were homogenously distributed with polydispersity index below 0.3 indicating stability, particle size in the range 120- 140 nm would help in improving the permeation and enhancing accumulation in the skin layers which is the ultimate requirement for treating psoriasis.
Further, in vivo investigations of the selected formulations will prove the suitability of such delivery systems for prolonged action of the topically applied anti-psoriatic agent, with lesser side effects than the conventional formulations [22].
We would like to acknowledge Indian Council for Medical Research (ICMR) for providing Senior Research Fellowship. We also thank Principal and Management of Al-Ameen College of Pharmacy, India for all the support rendered to carry out the work. We are very grateful to the Principal, Bharati Vidyapeeth University, Poona College of Pharmacy, for their support rendered for the rheological studies.
Download Provisional PDF Here
Article Type: Research Article
Citation: Vanaja K, Shobha Rani RH, Narendra C (2016) Application of Statistical Designs in the Formulation of Lipid Carriers of Methotrexate. J Drug Res Dev 2(2): doi http://dx.doi.org/10.16966/2470-1009.113
Copyright:© 2016 Vanaja K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access