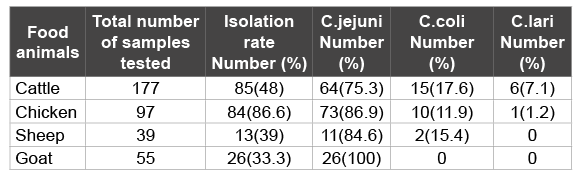
Table 1: Prevalence of Campylobacter species in feces/rectal swabs of 368 various food animals in Nuer zone, southwest, Ethiopia (April 2014 to February 2015)


Abdulhakim Abamecha1* Getahun Assebe2 Belay Tafa3 Beyene Wondafrash4
1Department of Biomedical Sciences, Faculty of Public Health and Medical Sciences, Mettu University, Mettu, Ethiopia*Corresponding author: Abdulhakim Abamecha, Department of Biomedical Sciences, Faculty of Public Health and Medical Sciences, Mettu University, Mettu, Ethiopia, E-mail: abdulhakimabamecha@gmail.com
Background: Campylobacters are the most common food borne zoonotic pathogens isolated from stools of patients with gastroenteritis worldwide. Despite Campylobacters with resistance to antimicrobial agents have been reported worldwide, the situation seems to deteriorate more rapidly in developing countries, where there is widespread and uncontrolled use of antibiotics. The aim of this study was to isolate and identify Campylobacter species and determine their antimicrobial resistance patterns from various food animals.
Methods: A cross sectional study was conducted in Lare district, Nuer Zone from April 2014 to February 2015. Isolation and identification of Campylobacter species from feces of various food animals were performed using standard bacteriological procedures. Antimicrobial resistance tests were performed using Kirby-Bauer disk diffusion method. Data were cleaned and entered into a computer and statistical analysis was performed using SPSS version 20. Chi-square and Fisher’s exact tests were used to test the differences between proportions, and 𝑃 value less than 0.05 was considered statistically significant.
Results: Out of 368 fecal samples processed, the rate of recovery of Campylobacters was 208 (56.5%); of which 174 (83.7%) were found to be C. jejuni, 27(12.9%) were C. coli and 7 (3.4%) were found to be C. lari. The overall antibiotic resistance profile showed that 46.6-82.2% of resistance was observed in C. jejuni and 0-29.6% of resistance was observed in C. coli isolates. The rate of multi-drug resistance was between 46.6-80.5% among C. jejuni. Multidrug resistant strains among C. coli were not observed.
Conclusions: The presence of drug resistant strains among the isolates revealed that humans are at risk from a variety of sources with potential for severe disease consequences. Therefore, the development of antibiotic-resistant strains needs better surveillance programs and monitoring of the use of antimicrobials in veterinary medicine in the area.
Campylobacter species; Antibiotic resistance; Food animals; Lare district; Gambella; Ethiopia
Campylobacters are Gram negative, non-spore forming, slender, spiral to curved rod-shaped bacteria that are commonly present in the intestinal tracts of domestic and wild animals [1]. Campylobacter species are classified “thermophilic” since they grow between 37 and 42°C, but incapable of growth below 30°C (absence of cold shock protein genes which play a role in low-temperature adaptation), with an optimum temperature of 41.5°C [2]. It is the leading human diarrheal pathogen in both developed and developing countries. Annually, approximately 400 million documented cases occur worldwide, resulting mainly from contamination of poultry or other meats, raw milk, other milk products and surface water [3,4]. Due to under-reporting, true number of cases is estimated to be up to 10 times higher than the documented case numbers [4]. In the United States, Campylobacters are ranked fourth among top five pathogens in causing food borne infections and is estimated to cause more than 9.4 million cases of Campylobacteriosis each year [5].
In developing countries, most of the infection is food borne primarily due to consumption of unpasteurized milk, contaminated water and meat especially poultry meat, rather than human to human transfer [6]. Cross contamination of ready to eat foods during preparation by food handlers as well as direct contact with food animals have also been identified [7,8]. Species of Campylobacter responsible for food poisoning are C. jejuni, C. coli, C. lari, and C. upsaliensis. The most common species associated with human Campylobacteriosis are C. jejuni and C. coli, which, together, cause around 95% of all Campylobacter infections [9,10].
Campylobacter causes an acute self-limited disease characterized by diarrhea, fever and abdominal cramps [11]. Extraintestinal manifestations are rare and may include meningitis, endocarditis, septic arthritis, osteomyelitis, and neonatal sepsis [6]. The most important post infectious complication of C. jejuni is Guillian Barre Syndrome (GBS) and Miller Fischer Syndrome which is an acute demylenating disease of peripheral nervous system [12].
Treatment with antibiotics for uncomplicated Campylobacter infection is rarely indicated. However, antimicrobial resistance to clinically important drugs used for treatment (especially macrolides and fluoroquinolones) is increasingly reported for Campylobacters [13]. There is evidence that patients infected with antibiotic-resistant strains suffer worse outcomes (invasive illness or death) than those infected with sensitive strains [14]. There is growing scientific evidence that the use of antibiotics in food animals, particularly in developed countries, leads to the development of resistant pathogenic bacteria that can reach humans though the food chain [15-17]. This underlines the need to limit the use of antimicrobials in veterinary practice to limit the occurrence of resistance.
The risks to human health vary between the different animal species and different countries and depend on variations in food preparation and consumption patterns. A reduction of the overall Campylobacter spp. burden in the food chain will result in a reduction in the number of cases of disease [18]. The few reported studies of Campylobacter spp. as human enteric pathogens in Ethiopia showed isolation rates ranging from 13.6% to 16.7% [19-21] and 39.6% from apparently healthy food animals [22]. Several countries have reported the epidemiology of Campylobacter in different wild and domestic animals [23-26]. The absence of national surveillance program, limited routine culture availability for the isolation of Campylobacter species at clinical and research settings, and the need for selective media and unique growth atmosphere make it difficult to give an accurate picture of the burden of disease in Ethiopia. This fact indicates that Campylobacter as a zoonosis is not given appropriate weight and consideration. In Ethiopia epidemiological data about the prevalence and antimicrobial susceptibility patterns of Campylobacter spp. are restricted to strains from clinical samples isolated from children with gastroenteritis [19-21]. There is neither an official surveillance nor monitoring system for the presence of Campylobacter in animals, nor for the use of antimicrobials in veterinary medicine. Therefore, the objective of this study was to investigate the prevalence and antimicrobial resistance patterns of Campylobacter species from intestinal tracts of various food animals in Nuer zone, Gambella, Southwest Ethiopia.
Nuer Zone is one of the three zones of the Ethiopian Region of Gambella. This zone is bordered by South Sudan on the south, west and north, by Anuak Zone on the southeast. Livestock product is the primary source of food and income in the Zone.
A cross sectional study was conducted at Lare district, Nuer zone from April 2014 to February 2015.
A single population proportion formula was employed to determine sample size using prevalence rate of 39.6 % from previous study [22] at 5% level of significance giving a total size of 368. One hundred seventy seven (177) cattle, 97 chickens, 39 sheep and 55 goats from Lare district were included in the study. Approximately 1-5 grams of fecal samples were obtained using direct rectal retrieval or rectal swab systems. The samples were taken with a sterile cotton swab moistened in nutrient broth and placed in Carry-Blair Transport medium (Oxoid Ltd, Basingstoke, Hampshire, England), and transported overnight on ice packs to Jimma University Medical Microbiology Laboratory.
The fecal samples/swabs were processed immediately upon arrival using aseptic techniques. The collected fecal samples/swabs were homogenized in Preston enrichment broth base containing Campylobacter selective supplement IV (HiMedia Laboratories, Mumbai, India) and 5% (v/v) defibrinated sheep blood. After inoculation at 42°C for 24 h under a microaerophilic atmosphere provided by gas generating sachets containing 5% O2 , 10% CO2 , and 85% N2 (Campy-Gen; Oxoid Ltd.), 0.1 mL of the enrichment broth was then streaked onto Campylobacter selective agar base (HiMedia Laboratories, Mumbai, India) supplemented with an antibiotic supplement for the selective isolation of Campylobacter species (HiMedia Laboratories, Mumbai, India) and 5% (v/v) defibrinated sheep blood and incubated at 42°C for 48 h under the same condition. Suspected Campylobacter colonies from each selective agar plate were subcultured and identification of a presumptive Campylobacter species was performed using standard bacteriological methods. For confirmation and differentiation of Campylobacter species, gram staining, production of catalase, oxidase, hippurate hydrolysis, and resistance to cephalothin and nalidixic acid were used [27-29].
Antimicrobial susceptibility tests were performed using KirbyBauer disk diffusion technique. Mullen-Hinton agar supplemented with 5% sheep blood was prepared. A 0.5 McFarland turbidity standard equivalent bacteria suspension for inoculation was prepared and inoculated. Antimicrobial disks were applied and the plate was incubated at microaerophilic atmospheric condition at 37°C for 48 hours. The following antimicrobials were used with their respective concentrations in parenthesis: ampicillin (AMP, 10 μg), chloramphenicol (C, 30 μμg), erythromycin (E, 15 μg), clindamycin (DA, 2 μg), gentamicin (CN, 10 μg), ciprofloxacin (CIP, 5 μg), norfloxacin (NOR, 10 μg), tetracycline (TE, 30 μg), cephalothin (KF, 30 μg), and nalidixic acid (NA, 30 μg). All the discs were from Oxoid Ltd. Company, England, UK. After 48 hours of incubation, the inhibition zones were measured to the nearest millimeter using a graduated ruler. The diameters of inhibition zones were measured around the disks and interpreted on the bases of CLSI 2011 interpretive criteria for Enterobacteriaceae to classify as sensitive, intermediate, or resistant [30] as described by others [1,31]. Campylobacter jejuni (ATCC 700819), Campylobacter coli (ATCC 33559), Staphylococcus aureus (ATCC 25923), Enterococcus faecalis (ATCC 29212), Pseudomonas aeruginosa (ATCC 27853), and E.coli (ATCC 25922) were used as control strains.
Data were cleaned and entered into a computer and statistical analysis was performed using SPSS version 20. The study findings were explained in tables. Chi-square and Fisher’s exact tests were used to test the differences between proportions, and 𝑃-value less than 0.05 was considered statistically significant.
Three hundred sixty eight (368) fresh faecal samples/rectal/cloacal swab samples were analyzed in the study. The rate of recovery of Campylobacter was 56.5% (208 out of 368) for all food animals. The number and percentage of Campylobacter strains isolated from each food animals were: cattle, 64/15/6; chicken, 73/10/1; sheep, 11/2/0; and goat, 26/0/0 respectively. All isolates found in goats were C. jejuni (100%), (Table 1).

Table 1: Prevalence of Campylobacter species in feces/rectal swabs of 368 various food animals in Nuer zone, southwest, Ethiopia (April 2014 to February 2015)
According to the statistical analysis, the percentage of Campylobacter strains isolated from different food animals were statistically significant (p-value <0.05%).
Among the 208 thermophilic Campylobacter strains isolated from various food animals, 174 (83.7%) were found to be C. jejuni, 27(12.9%) were C. coli and 7 (3.4%) were found to be C. lari. The number of C. jejuni, C. coli and C. lari isolated per food animal species were: cattle, 64/15/6; chicken, 73/10/1; sheep, 11/2/0; and goat, 26/0/0 respectively. All isolates found in goat were C. jejuni (100%), (Table 1).
The antimicrobial resistance profile of C. jejuni and C. coli strains from food animals are shown in (Table 2). The rate of resistance of C. jejuni showed; ampicillin, chloramphenicol, clindamycin, gentamicin, ciprofloxacin, norfloxacin, tetracycline, cephalotin, nalidixic acid and erythromycin were (46.6%), (61.5%), (54.6%), (50%), (80.5%), (64.9%), (82.2%), (100%), (3.8%) and (60.3%), respectively. The rate of resistance of C. coli showed; ciprofloxacin and cephalotin were (29.6%) and (100%), respectively. The rate of multiple resistances among Campylobacter spp is shown in (Table 3). According to the different antibiotic groups, the frequencies of multidrug resistant strain (non-susceptible to ≥ 1 agent in ≥ 3 antimicrobial categories) were between 46.6-80.5% among C. jejuni. The frequencies of multidrug resistant strain among C. coli were 0%. Cephalothin was not considered as a multidrug resistant variant property as most Campylobacters are inherently resistant to this agent.
The occurrence of human Campylobacter gastroenteritis has largely been attributed to the consumption of contaminated food of animal origin [6]. In the present study, the prevalence of Campylobacter spp. in cattle fecal samples was found to be 48%. Our finding was inconsistent with Campylobacter isolated from fecal samples collected from cattle that previously were 34% [32], 27.9% [33]and 23.4% [34]. These differences in the prevalence of cattle associated Campylobacter can be attributed to several factors, including isolation methods, sample size and geographical location [11]. Out of 85 tested isolates the prevalence of C.jejuni was 75.3% that was higher than 17.6% C. coli and 7.1 C. lari. Our results were in harmony with Nielsen et al. [35] who found 90.9% of the isolates from fecal samples of cattle were Campylobacter jejuni and 6.8% were C. coli, Cakmak and Erol [36] identified 40.4% C. jejuni and 4.1% C. coli in Turkey meat samples. Our study reveals that healthy cattle are considered as reservoir for a number of thermophilic Campylobacter species, highlighting the importance of non-poultry farms as possible sources of Campylobacter infection. The high prevalence of C. jejuni in cattle should be of special concern and care should be taken to limit its spread during milking, slaughtering and dressing.
The reported prevalence of Campylobacter bacteria in broiler flocks ranged between 35-57% in Europe [37] and 64-100% in Africa [26,38,39]. In this study, 86.6% isolation rate of thermophilic Campylobacter is observed in chickens, which is higher than the findings from previous study in Ethiopia (68.1%) [22]. Among the isolates, C.jejuni predominantly found (86.9%) followed by C. coli (11.9%) and C. lari (1.2%) (Table 2). Similar results have also been reported in other countries indicating a higher prevalence of C. jejuni than C. coli in poultry farms [40].
These results indicated that chickens raised without sanitary attention may harbor thermophilic Campylobacter species, especially C. jejuni. One might consider this an important risk factor, since there is a correlation between the presence of these bacteria in poultry products and its concentration in the intestinal tract [41].
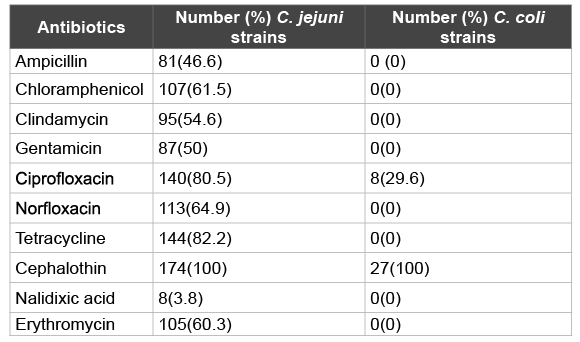
Table 2: Resistance to 10 antibiotics in 174 C. jejuni and 27 C. coli strains isolated from food animals in Nuer Zone, Gambella, southwest, Ethiopia(April 2014 to February 2015)
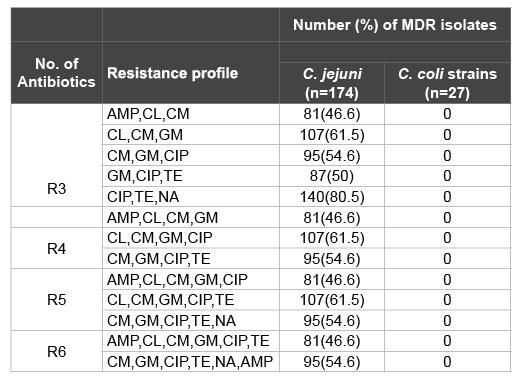
Table 3: Multidrug-resistance patterns of C. jejuni and C. coli strains isolated from various food animals in Nuer Zone, Gambella, southwest, Ethiopia (April 2014 to February 2015)
MDR: non-susceptible to ≥ 1 agent in ≥ 3 antimicrobial categories
R3: Resistance to three antibiotics; R4: Resistance to four antibiotics;
R5: Resistance to five antibiotics; R6: Resistance to six antibiotics; AMP:
Ampicilin TE:Tetracycline; CIP: Ciprofloxacin; CL: Chlormphenicol; ERY:
Erythromycin; GM: Gentamycin; CM: Clindamycin; NA: Nalidixic acid
The prevalence of Campylobacter spp. in feces of sheep investigated was 39% which is comparable to the isolate rates of 38% reported by kassa et al. [22]. However, the present finding was higher than the rates of 11.9% recorded by reported by Acik and Cetinkaya [42], and 10.6% by Chanyalew et al. [43]. Among the thermophilic Campylobacter species isolated 84.6% were C. jejuni and 15.4% were C.coli. This was in agreement with those findings reported by Kassa et al. [22], Salihu et al. [44], and Rahimi et al. [45]. This implies that C. jejuni is the most common Campylobacter species in sheep in Ethiopia.
In this study, 33.3% (26 out of 55) of the goats sampled were found to harbor Campylobacter isolates in their faeces. In a study done in Sokoto State, Nigeria, on 1312 faecal samples, over a period of 2 years, Salihu et al. [44] found 20% of the faecal samples to be positive for Campylobacter. This high prevalence of Campylobacter in goat is of serious concern in view of the high rate of consumption of goat meat and row goat milk in most African countries. C. jejuni is the main causative agent of food-borne gastroenteritis in humans and also causes a variety of diseases, such as enteritis, abortion, septicaemia and mastitis in animals [46]. In this study, all isolates found in goat were C. jejuni (100%). This indicates that goats can be regarded as a potential reservoir for human Campylobacteriosis.
Antibiotic resistance in Campylobacter is emerging globally and has already been described by several authors and recognized by the WHO, as a problem of public health importance [5,47,48]. Campylobacter species resistant to antibiotics (C. jejuni, and C. coli) can be transferred from different sources to humans. This situation, alarmingly, announces the need to perform antimicrobial sensitivity tests for Campylobacters. Macrolids and fluoroquinolones are usually considered the drugs of choice for treatment of food-borne Campylobacteriosis [11,41,49]. Since C.jejuni and C.coli demonstrate different susceptibility profiles, it is important to differentiate Campylobacter at the species level, and to provide antimicrobial susceptibility data for each species, in order to monitor the trend of antimicrobial resistance among Campylobacter isolates and to ensure effective treatment of Campylobacter infections. In the present study, most of the isolates were found to be resistant to the fluoroquinolone class of antibiotics, out of 174 C. jejuni isolates, 140 (80.5%) were resistant to ciprofloxacin, and 113(64.9%) were resistant to Norfloxacin. On the other hand, out of 27 C. coli isolates, 8(29.6) were resistant to ciprofloxacin, but no resistant strain to Norfloxacin. The resistance to erythromycin was reported to be 105(60.3%) among C. jejuni and none among C. coli isolates. This finding is a cause of concern, because the drugs of choice for treatment of food-borne Campylobacteriosis especially C.jejuni totally abolished. In such instances, controlling the spread of these organisms becomes of paramount importance.
Alternative antibiotic in the treatment of Campylobacterosis showed high rate of resistance. Out of 174 C. jejuni isolates, resistance to Ampicillin was observed in 81(46.6%), 107(61.5) to Chloramphenicol, 95(54.6%) to Clindamycin, 50(87%) to Gentamicin, 144(82.2%) to Tetracycline, 174(100%) and 8(3.8%) to Nalidixic acid. All Campylobacter strains isolated in the current study were resistant to cephalothin as most of these species are inherently resistant to the drug.
Several investigators have reported the increasing incidence of human C. jejuni and C. coli infections in many parts of the world for the last decade with higher multidrug resistance [49-51]. Multidrug resistance has been observed in most of the Campylobacter isolates in the present study. Out of 174 C. jejuni isolates, 46.6-80.5% isolates were multidrug resistance C. jejuni. These findings are also in agreement with the observations of several other researchers [11,52,53].
We can conclude from our study that thermophilic Campylobacters, C. jejuni and C. coli/C. lari are very frequent among food animals in Nuer Zone, Ethiopia, suggesting possible risks of infection to people through consumption of contaminated animal products or by direct contact with infected animals. Since the available evidence shows that food animals constitute a major reservoir for these organisms, interruption of transmission to human beings from these sources should be given a high priority. Awareness of the necessity for hand washing after contact with animals and their products and the importance of proper cooking and handling of foods of animal origin are probably as important in preventing C. jejuni/coli infections. Pasteurization of milk, chlorination of water supplies and proper cooking of foods readily kill these organisms.
The presence of drug resistant strains among the isolates reveals that humans are at risk from a variety of sources with potential for severe health consequences, Therefore, the development of antibiotic-resistant strains, call for surveillance and monitoring the use of antimicrobials in veterinary medicine to detect emerging resistance and to prevent the spread of antibacterial-resistant Campylobacter strains.
The authors would like to acknowledge Gambella University and Mettu University for the financial support. They would also like to thank Gambella Agricultural and Rural Development Bureau and the Lare district officials for allowing them to undertake the study. Finally, the authors acknowledge Jimma University for allowing the Laboratory set up.
The authors have no financial or other conflict of interest to declare in relation to this manuscript.
Abdulhakim Abamecha designed the study and carried out the laboratory works and analysis, and drafted the manuscript. Beyene Wonafrash, Getahun Assebe, and Belay Tafa participated in the design of the study and helped to draft the manuscript. All authors have read and approved to the final version of the manuscript.
Download Provisional PDF Here
Article Type: Review Article
Citation: Abamecha A, Assebe G, Tafa B, Wondafrash B (2015) Prevalence of Thermophilic Campylobacter and their Antimicrobial Resistance Profile in Food Animals in Lare District, Nuer Zone, Gambella, Ethiopia. J Drug Res Dev 1(2): doi http://dx.doi.org/10.16966/2470-1009.108
Copyright: © 2015 Abamecha A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access