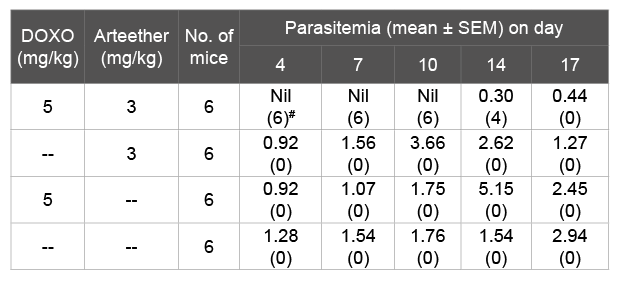
Table 1: Response of DOXO in combination with ART against PvAR strain in Swiss mice
# Number in parenthesis indicates mice showing nil parasitemia

Ramesh Chandra* Puri SK
Division of Parasitology, Central Drug Research Institute, India*Corresponding author: Ramesh Chandra, Division of Parasitology, CSIR-Central Drug Research Institute, Lucknow, Uttar Pradesh 226031, India, E-mail: rameshcdri1@gmail.com
Artemisinin based combination therapies may effectively reverse the resistance in malaria parasite by reversal agents. Here, we are first time showing arteether (ART) drug resistance reversal in Plasmodium vinckei by using Doxorubicin (DOXO). We hypothesized that oxidative stress catabolism may interfere with DOXO, resulting increased the efficacy of ART. We show the synergetic effect of DOXO with ART by complete suppression of parasitemia till day 10 post treatment. In addition, results also showed higher Activity Enhancement Index (AEI) of ART. These studies propose the possibility of ART drug combination that may be helpful in reducing the chance of resistance development.
α/β Arteether; Activity enhancement index; Doxorubicin combination; Plasmodium vinckei; Resistance reversal
Artemisinin and its derivatives are truly remarkable drug and the standard of care for malaria over about quarter a century. These are the most effective antimalarial drugs that act against the chloroquine resistant P. falciparum [1]. The mechanism of action, involves the breaking of endoperoxide bridge and generating a highly reactive free radical by activation of parasitic iron. The activated endoparasites form a covalent bond with parasite proteins killing the parasite [2,3]. Artemisinin-based combination therapies (ACTs) [4-6] have now become the standard of care for malaria chemotherapy, against malarial parasite resistance to existing antimalarial drugs [7,8]. α/β arteether is a semi synthetic derivative of artemisinin, acting as the blood schizontocidal agents at the erythrocytic stage of malaria in blood. ART is registered in India [9] and is now being used for second line of treatment for chloroquine-resistant P. falciparim malaria including cerebral malaria [10-12]. This is most effective against chroroquine resistance against P. yoelii [13] P. vinckei [14] and P. falciparum [1,11,12]. ART based combination have been studied with Curcumin in P. berghi [15]. ART and sulfadoxine-pyrimethamine for P. falciparum is the most effective combined treatment [16]. Limited studies have been carried out that proposes ART drug resistance reversal, which in turn can be more promising. Here, we first time showed the ART drug resistance reversal in P. vinckei by using anticancer drug Doxorubicin (DOXO). The DOXO is an anthracycline antibiotic that has been employed as anticancer drugs and is routinely being used in the clinic as chemotherapeutic agents [17]. The mechanism of action of DOXO involves oxidative stress, inhibition of DNA polymerases, DNA topoisomerase II, and DNA methyltransferase [18-20].
Swiss albino mice weighing 24-26 g were obtained from the Division of Laboratory Animals, Central Drug Research Institute, Lucknow, India. The mice were sheltered in the animal facility at the Institute and maintained on commercial pellet diet and water ad libitum under standard housing conditions. Ethical guidelines on handling, care and use of experimental animals were followed during the conduct of the study. We have used stable PvAR parasite for this study as described before [21,22]). The stable selected ART resistant Plasmodium vinckei (PvAR) was maintained by drug pressure, which showed >24- fold resistance at the time of study [23,24]. However, inoculum for each experiment was obtained from infected mice left untreated for one passage.
Resistance reversal studies were performed by pharmacological agents in combination with ART. Six mice per drug combination were inoculated by an intraperitonial (i.p.) injection of 1 × 106 infected erythrocytes [24]. The treatment was started 4 h later and continued for the subsequent 4 days [24]. DOXO was obtained from Dabur Pharma Ltd, India. The antimalarial drugs artemisinin derivative α/β ART obtained from Medicinal Chemistry Division, CDRI. ART was dissolved in ground nut oil and administrated by intramuscular (i.m.) injection. While partner drugs were diluted in aqueous solution and administrated by i.p. routes. Parasitemia was recorded on days 4, 7, 10, 14 and 17 and compared to the group treated with ART alone.
Group of mice was inoculated with 1 × 106 parasitized cells and treatment was administered for 4 days. Microscopic slides were observed on day 4. The suppression in parasitemia was counted with comparison with vehicle control. ED50/ED90 value was calculated by non-linear regression analysis [23]. Parasitemia was monitored in thin blood smears, fixed in methanol and stained with Giemsa (Merck, Germany) for 30 min. The slides were washed in tap water, dried and examined in at least 20 fields per slide using the 100X microscope to determine the percentage of infected erythrocytes [23].
ctivity enhancement index (AEI) was determined for synergetic effect of partner drug as described before [25,26]), which was calculated as follows: ED90 of ART alone/ED90 of ART in combination with partner drug. An AEI of >1 indicates activity enhancement, and an AEI of <1 indicates activity reduction. An AEI of less than 2.0 was considered insignificant.
Our previous study has shown that higher antioxidant enzyme and glutathione may be correlated with ART resistance in P. vinckei [23,24]). We hypothesized that glutathione and oxidative stress catabolism may interfere with DOXO, resulting in an increased efficacy of ART in PvAR parasite. To investigate the combination effect of antitumor drug DOXO, we have injected it to infected mice alone in combination with ART for 4 days to PvAR infected mice. DOXO was treated to infected mice at doses level 5 mg/kg in combination with 3 mg/kg of ART [23]. Parasitemia was recorded on days 4, 7, 10 and 14 and compared with group treated with ART alone. The result showed that none of the mice developed parasitemia up to day 10 in combine group and 2 mice became patent on day 14. All mice became patent on day 10 (Table 1). However, mice treated with DOXO or ART alone show the parasitemia on day 4. This result indicates that DOXO has synergetic effect on mice treated with ART as evidenced by their extended day of suppression of parasitemia as compared to the group treated alone (Table 1).
To evaluate the AEI of ART, first we determined ED50/ED90 ART alone for which results are 4.49 and 17.23 mg/kg respectively (Table 2). We next evaluated the ED10/ED50/ED90 of DOXO, at four serial dose levels i.e. 4, 2, 1, 0.5 mg/kg and vehicle control for four consecutive day’s treatment to each group. The results showed that ED10/ED50/ED90 values for DOXO against PvAR strain are 0.39, 2.02 and 3.65 mg/kg respectively (Table 2). Next we evaluated ED50/ED90 of ART in combination with DOXO. We have chosen two sub-curative dose of DOXO 0.4 and 2 mg/kg, which are ED10 and ED50 if used alone respectively (Table 2). Each group was infected with PvAR parasites and treated with ART at dose levels 10, 3, 1, 0.3 & 0.1 mg/kg for four consecutive days in combination with 0.4 or 2 mg/kg DOXO. The results conclude that ED50/ED90 of ART was reduced from 4.49 and 17.23 mg/kg to 3.19 and 7.92 at dose 0.4 mg/kg DOX, and 0.07 and 1.90 at dose 2 mg/kg DOXO (Table 2). Activity Enhancement Index (AEI) of ART was significantly higher 2.17 and 9.07 combination with DOXO at 0.4 and 2 mg/kg respectively (Table 2).
These results further suggested the synergistic effect of anti-malaria activity in P. vinckei by DOXO and ART combination, since none of the mice developed infection up to day 10 and higher EAI index of ART. The mechanism of action artemisinin, involves the breaking of endoperoxide bridge and generating a highly reactive free radical that kills malaria parasites [27,28],). Our previous study has shown that higher antioxidant enzyme, glutathione may be correlated with ART resistance in P. vinckei [24,23]. The DOXO enhanced the oxidative stress and showed the synergetic effect with artemisinin in P. falciparum [29,30]). Dihydroartemisinin is derivative of artemisinin that also has synergistic anti-cancer activity in the combination with doxorubicin in breast cancer cells [31]. The DOXO lowered GSH or GSH reductase levels in-vitro cultured rat heart cells [32]. This indicates indicating that ART-DOXO exerts their chemotherapeutic effect by increasing the oxidative stress. This synergistic effect can be largely explained by the fact that ART and DOXO use killing of resistant parasite [33]. Overall, this study give better idea to the possibility of ART drug combination that may be helpful in prolonging the life of drug and a promising lead to reduce the chance of resistance development of artemisinin and its derivative.

Table 1: Response of DOXO in combination with ART against PvAR strain in Swiss mice
# Number in parenthesis indicates mice showing nil parasitemia
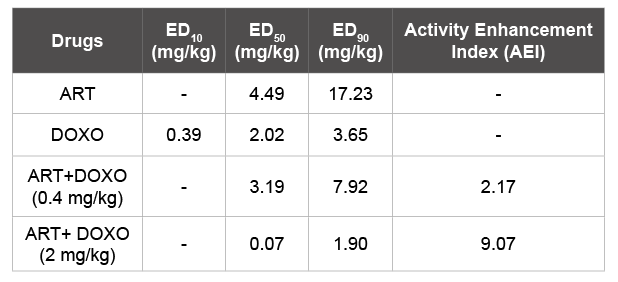
Table 2: ED50/ED90 of ART and DOXO alone/combination and also showing Activity Enhancement index of ART
The authors are grateful to the Director of the CDRI for continued support and encouragement. Technical assistance rendered by Mr. K.K. Singh is gratefully acknowledged. The award of a research fellowship to one of the authors (RC) from CSIR, New Delhi, is also acknowledged.
Download Provisional PDF Here
Aritcle Type: Short communication
Citation: Chandra R, Puri SK (2015) Reversal Effect of Doxorubicin on Arteether Resistance in Rodent Malaria Parasite Plasmodium vinckei. J Drug Res Dev 1(1): doi http://dx.doi.org/10.16966/2470-1009.104
Copyright: © 2015 Chandra R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access