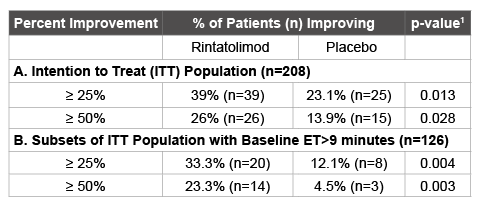
Table 1: Analysis of Percentage of CFS/ME Patients Improving ET by at least 25% and 50% from Baseline

David R Strayer1 Bruce C Stouch2 Staci R Stevens3 Lucinda Bateman4 Charles W Lapp5 Daniel L Peterson6 William A Carter1 William M Mitchell7*
1Hemispherx Biopharma, Inc., Philadelphia, Pennsylvania, United States of America*Corresponding author: William M. Mitchell, Department of Pathology, Microbiology & Immunology, Vanderbilt University, Nashville, TN 37205, USA, Tel: 615-322-3238; E-mail: bill.mitchell@vanderbilt.edu
Background: Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) is a debilitating disease of unknown pathogenesis consisting of a variety of flu-like symptoms including severe fatigue. Initial analysis of the use of rintatolimod (Poly I: Poly C12U), a selective TLR3 agonist, in a Phase III, double-blind, randomized, placebo-controlled trial of CFS/ME demonstrated statistical significance (p<0.05) in the reduction of fatigue as measured by exercise tolerance (ET) as the primary endpoint using a modified Bruce protocol with reduced physical exertion in patients with severe CFS/ME as defined by a Karnofsky performance score (KPS) of 40-60.
Methods and Findings: In order to better identify responders to rintatolimod, primary and secondary endpoints have been reexamined post hoc as a function of a pre-specified study baseline ET duration >9 minutes. Analysis of improvement in exercise performance at the ≥ 25% and ≥ 50% levels using ET at 40 weeks compared to baseline was performed for the intent-to-treat (ITT) population (n=208) using the pre-specified baseline exercise stratum (baseline ET duration >9 minutes). For this subset of patients (n=126), 33% (n=20), and 12% (n=8) of rintatolimod vs. placebo patients, respectively, improved ET duration by ≥ 25% (p=0.004) while 23% (n=14) compared to 4.5% (n=3) of rintatolimod vs. placebo patients, respectively improved ET duration by ≥ 50% (p=0.003). This corresponds to increases of ≥ 186 and ≥ 373 seconds for patients receiving rintatolimod, respectively, at ≥ 25% and ≥ 50% improvement responses. A frequency distribution analysis of ≥ 25% improvement, <25% change, and ≥ 25% deterioration in ET from baseline at 40 weeks for the baseline >9 minutes cohort showed net improvement to be 18.3% for the rintatolimod cohort vs. 4.6% deterioration for placebo (p=0.015). A continuous responder analysis using 5% increments from ≥ 25% to ≥ 50% provided a robust clinical enhancement in ET effect in the rintatolimod cohorts as compared to placebo. The KPS and Vitality (SF-36 subscale) quality of life secondary endpoints demonstrated similar clinically significant improvements for the rintatolimod cohort as a function of the same ET dichotomization. Rintatolimod was generally well-tolerated in this CFS/ME population.
Conclusions: Using a modified Bruce ET protocol with reduced physical exertion allowed clear identification of patient responders to rintatolimod with severe CFS/ME syndrome. Rintatolimod produced significant enhancement in ET and quality of life indicators in patients able to complete >9 minutes in a modified Bruce ET test. Rintatolimod also reduced deterioration in ET compared to placebo in patients with the poorest initial ET. Exercise endurance >9 minutes in a Bruce protocol modified for patients with CFS/ME provides a method to identify patients most likely to respond to rintatolimod.
Rintatolimod; PolyI:C12U; Chronic fatigue syndrome; Myalgic encephalomyelitis; Phase III clinical trial; Exercise tolerance; Karnofsky Performance Score (KPS); Short form 36; Quality of life
Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) is a debilitating disorder often characterized by an incapacitating mental and physical fatigue that is not improved by bed rest and a combination of flulike symptoms [1-3]. Phase III clinical trial patients with severe CFS/ME demonstrated significant improvement in the primary endpoint, exercise tolerance (ET), following the systemic administration of rintatolimod, a selective TLR3 dsRNA agonist [4,5], twice weekly for 40 weeks compared to placebo [6]. Some patients dramatically responded to rintatolimod administration while others did not. Our hypothesis was that baseline exercise tolerance could be used to predict ET responses to rintatolimod. This report demonstrates that post hoc analysis of ET response reveals differential responses to rintatolimod that clearly identify three classes of patients. The first is defined by marked improvement in ET as well as secondary endpoints. The second class does not significantly respond efficaciously to rintatolimod. The third class, although deteriorating on rintatolimod, do so at a reduced rate compared to controls.
The study was a prospective, double-blind, Phase III trial with equal parallel cohorts conducted at 12 centers in the U.S. to evaluate the safety and efficacy of rintatolimod in CFS/ME (Trial Registration: ClinicalTrials.gov NCT00215800). The inclusion and exclusion criteria for study enrollment is detailed in Supplemental Table S1 and meets both the original [1] and revised [2] CDC clinical definitions of CFS. Study details including a flow diagram of all study patients can be found in the original study report [6]. The design of the study, including endpoints, was reviewed by the FDA prior to receipt of FDA authorization for the initiation of the study. A summary of the demographic characteristics of the trial is provided in Supplemental Table S2. Many of the CFS/ME patients were unable to physically perform the standard Bruce submaximal exercise protocol commonly used for the evaluation of cardiac function, so the primary endpoint was adapted to a change in ET from baseline to week 40 using a modified Bruce treadmill protocol for CFS/ ME patients (Supplemental Table S3). This protocol is similar in energy requirements to a modified Bruce protocol used commonly for the elderly [7] that severely compromised CFS/ME patients could perform without risk of injury. Secondary endpoints analyzed as a function of ET dichotomization were the performance related quality of life monitors, Karnofsky Performance Score (KPS) (Supplemental Table S4) and Vitality (SF-36 subscale). Patients were stratified according to their treadmill duration (≤ 9 minutes vs. >9 minutes) and randomized to receive either rintatolimod or placebo.
Data analyses used SAS (Version 9.2) statistical software (Cary, NC). The reported probability values from all statistical analyses were two-sided. The sample size for this clinical investigation was based on detecting a difference in the intra-patients changes in ET (seconds) between the randomized treatment groups using a 2-tailed test and type 1 error rate of 5%. The primary endpoint (intra-patient changes in treadmill duration, week 40 minus baseline) was analyzed using a onefactor (treatment assignment) analysis of covariance test (ANCOVA) with the mean of two baseline ET tests serving as the covariate. Although the design of the study considered repeated measurements on the patients over time, the statistical model for evaluating efficacy was predicated on a landmark analysis based on the intra-patient changes at week 40. The 2-sample t-test was used to compare baseline ET between the two randomized treatment groups. The proportion of patients who achieved a ≥ 25% and ≥ 50% increase in ET at week 40 (intra-patient changes or within subject changes) was compared between randomized treatment groups using a two-tailed chi-square test. Defining what constituted clinically meaningful intra-patient improvement in ET was based on intra-patient variability with regard to two ET examinations performed during baseline. The variability of treadmill testing showed that a 25% minimum level exceeded intra-patient variability in over 90% of the patients. A ≥ 25% improvement or deterioration is also supported by the medical literature [8-11]. Twice the 25% minimum level of change or a ≥ 50% change in ET was considered a major clinical response [8,10]. A continuous responder analysis was performed using 5% increments from ≥ 25% to ≥ 50% ET improvement. A frequency distribution of the number of patients with ≥ 25% improvement, <25% change, and ≥ 25% worsening in ET from baseline at 40 weeks was analyzed using probability values derived from the 2-sided Chi-Square test, or 2-tailed Fishers’s Exact test (used if any cell had less than five observations). Secondary endpoints were analyzed based on the distribution of the dependent variable. The normality of the distributions was examined using the Shapiro-Wilk test.
The ITT population included all patients who received study drug and performed the ET study parameter at least once during the treatment phase. The last ET observation was used for patients who failed to complete the week 40 visit. A completer group, consisting of all patients who competed the 40 weeks of Stage 1, was also pre-specified in the protocol.
As previously reported, rintatolimod produced an objective improvement in ET in a Phase III clinical trial [6]. An intention to treat (ITT) analysis (n=208) of ET yielded an intra-patient, placebo-adjusted enhancement in mean ET at week 40 of 21.3% (p=0.047, ANCOVA with baseline as a covariate) with a 24.6% intra-patient, placebo-adjusted enhancement in mean ET for patients (n=194) who completed all 40 weeks (p=0.019). An independent statistical analysis of the result was conducted and the parametric p-value of 0.047 was confirmed with a non-parametric analysis yielding a value of p=0.013 using the van der Waerden rank order test. Table 1A illustrates the proportions of patients in the ITT population with increases from mean baseline ET duration at week 40 of at least 25% and of at least 50% were 1.7 and 1.9-fold greater for subjects randomized to rintatolimod than placebo, 39% versus 23% (p=0.013) and 26% versus 14% (p=0.028), respectively. Mean baseline ET levels for the rintatolimod (n=100) and placebo (n=108) cohorts of the ITT population were 576 and 588 seconds, respectively. Thus, ≥ 25% and ≥ 50% increases in ET for the patients receiving rintatolimod were ≥ 144 and ≥ 288 seconds, respectively. Table 1B shows the same analysis for the pre-declared stratification subset with baseline ET >9 minutes. The proportions of patients in this subset with increases from mean baseline ET duration at week 40 of at least 25% and of at least 50% were 2.8 and 5.2- fold greater for subjects randomized to rintatolimod than placebo, 33.3% versus 12.1% (p=0.004) and 23.3% versus 4.5% (p=0.003), respectively. Mean baseline ET levels for the rintatolimod (n=60) and placebo (n=66) cohorts of this stratification subset with baseline ET >9 minutes were 747 and 738 seconds, respectively. Thus, ≥ 25% and ≥ 50% increases in ET for these patients receiving rintatolimod were ≥ 186 and ≥ 373 seconds, respectively. The robustness of these dichotomized analyses is demonstrated at each 5% increment between ≥ 25% to ≥ 50% in Figure 1.
The two performance based secondary endpoints, (KPS) (Table S4) and Vitality are similarly affected by dichotomization as a function of ET improvement at <25% versus ≥ 25% (Table 2). Dichotomizing the rintatolimod treated ITT population based on significant clinical improvement ( ≥ 25%) at Week 40 in ET duration shows there is corresponding clinically significant improvements in secondary endpoints, KPS and Vitality for the ≥ 25% ET improving rintatolimod cohort compared to the <25% cohort.
As illustrated in Figure 2, the Vitality increased a clinically significant 14 points from baseline for rintatolimod patients with a ≥ 25% improvement in ET at week 40, while the minimum clinically important difference (MCID) is 5 points [8]. The change in Vitality score for the <25% cohort was 4.5 points and did not reach the MCID of 5 which is indicated by the red line in Figure 2. Vitality is one of the best SF-36 subscales for measuring the reduction in functioning seen in patients with CFS [9].

Table 1: Analysis of Percentage of CFS/ME Patients Improving ET by at least 25% and 50% from Baseline
1 Probability values derived from the Chi-square test or Fisher’s Exact Test if any cell had less than 5 observations
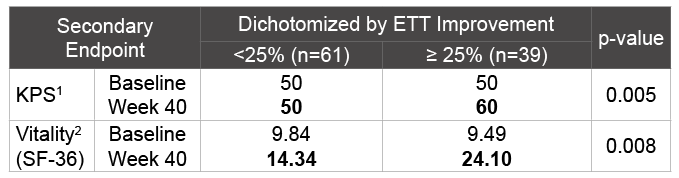
Table 2: Dichotomizing the Rintatolimod Treated ITT Population Based on Significant Clinical Improvement ( ≥ 25%) in ETT Duration at Week 40
1 Median with p-value based on Wilcoxon Two-Sample test (two-sided)
2 Mean with p-value based on 1-factor ANOVA model
The individual patient ET responses to rintatolimod compared to placebo for the ITT population is captured in Figure 3. Individual patient change in ET from baseline at 40 weeks is plotted from lowest to highest ET performance. There is a minimum of three different ET response cohorts- a high response cohort, a minimal response cohort, and a negative response cohort represented by approximately 1/3 of the total in each cohort. In the high response cohort there is a clear improvement in ET. The middle cohort represents minimal change between rintatolimod and placebo. The negative response cohort shows deterioration in ET performance in both rintatolimod and placebo patients. Nevertheless, rintatolimod clearly reduced deterioration in ET versus the placebo controls. This is presented in a quantitative fashion in Table 3 for the subset of the ITT population with an ET baseline >9 minutes on the Bruce treadmill protocol modified for CFS/ME. At both the ≥ 25% and ≥ 50% improvement levels there were more patients showing deterioration compared to improvement in the placebo groups compared to the rintatolimod cohorts. The net improvement for the ≥ 25% cutoff was 22.9% (p=0.015). At ≥ 50% a net 27.9% improvement was observed (p<0.001).
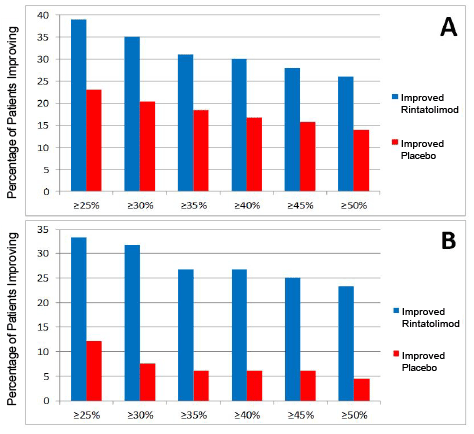
Figure 1: Robustness of dichotomized patient responses from ≥ 25% to ≥ 50% ET improvement at 5% increments; Panel A. ITT population. Panel B. protocol pre-declared ITT population subset with baseline ET duration >9 minutes.
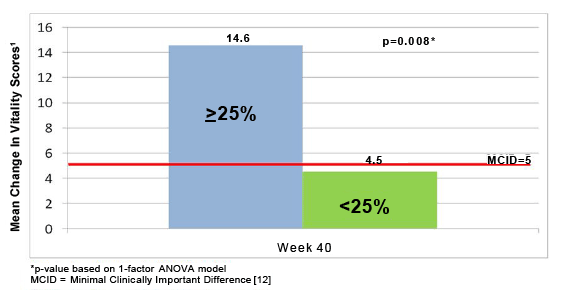
Figure 2: Vitality score changes from baseline for rintatolimod patients with a ≥ 25% improvement in ETT at week 40 vs. <25% improvement.
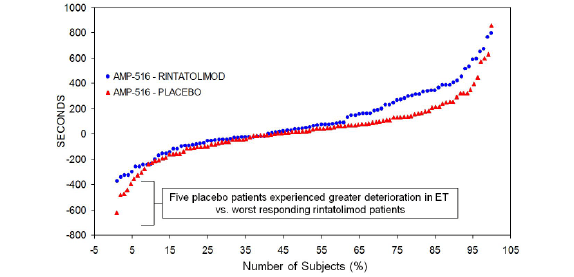
Figure 3: Plot of ET difference from baseline in seconds at 40 weeks treatment (ordinate) per each patient (abscissa).
Post hoc analysis of a phase III double-blind, randomized, placebo controlled clinical study of rintatolimod has demonstrated a subset of patients with a significantly improved quality of life and a method to identify the patients most likely to respond. In the cohort of patients able to exceed 9 minutes on the modified Bruce treadmill at baseline, the rintatolimod treated arm showed a 2-4 fold greater response rate compared to the placebo treated group (panel B of Figure 1). The intrapatient ET responses in the rintatolimod cohort versus placebo were rather evenly distributed over the entire range between ≥ 25% to ≥ 50% ET improvement using 5% increments for both the ITT population (panel A of Figure 1), as well as, those patients who were able to continue on the treadmill over 9 minutes at baseline (panel B of Figure 1). Although some rintatolimod patients deteriorated during the 40 week trial, this selective TLR3 agonist [4,5] reduced the frequency of ET deterioration compared to that observed in the placebo controls (Table 3). The spectrum of ET responses is plotted for each patient in Figure 3 as change from baseline in ET at 40 weeks for both the rintatolimod and placebo cohorts. The positive response in ET to rintatolimod is reflected in the upper 40% of the cohort matched responses on the right side in Figure 3. Patients receiving placebo deteriorated greater than the corresponding rintatolimod control patients as shown on the left side of Figure 3 since the rintatolimod frequency distribution function did not drop below that of placebo. This suggests that rintatolimod retards deterioration of CFS symptoms in nonresponders, which is also observed in Table 3. The KPS and Vitality Scores were similarly enhanced in the rintatolimod cohort of the ITT population with clinically significant (≥ 25%) ET improvement after 40 weeks of treatment (Table 2 and Figure 2). Importantly, these improvements, a 10 point increase in KPS and a 14.6 point increase in Vitality scores are both clinically relevant of significant changes that represent objective improvement in quality of life.
Rintatolimod has demonstrated statistical significance in improvement of primary endpoints in two randomized, double-blinded placebocontrolled clinical trials in patients with well-defined CFS/ME [6-14]. No other pharmaceutical agent is in advanced clinical development to our knowledge in this woefully disabling and neglected disease. This selective TLR3 agonist is clearly active in a subset of the ITT population producing ≥ 25% improvements in intra-patient ET responses. Rintatolimod also reduced deterioration in ET compared to placebo in patients who fail to improve physically
Our analysis of the differential responses to rintatolimod in patients with severe CFS/ME has identified a marker to help predict clinical response to rintatolimod. The primary endpoint was ET using a Bruce protocol modified for CFS/ME with energy expenditure less than that of the standard Bruce protocol for non-athlete cardiac stress test. Patients meeting strict diagnostic criteria for CFS/ME that can physically exceed 9 minutes duration on a Bruce exercise protocol modified for CFS/ME, have a better ET response to this TLR3 agonist than patients with a ≤ 9 minute duration.
A modified Bruce ET protocol allowed clear identification of a rintatolimod responder subset in patients with severe CFS/ME syndrome. Rintatolimod produced significant enhancement in ET and quality of life indicators in patients able to complete >9 minutes exercise. Rintatolimod also reduced deterioration in ET compared to placebo in patients with the poorest initial ET. Exercise endurance >9 minutes in a Bruce protocol modified for patients with CFS/ME provides a method to identify patients most likely to respond to rintatolimod.
We are appreciative for the independent statistical analysis and expertise provided by David A. Schoenfeld, Ph.D.
WMM is an independent Director of the BOD for HEM. WAC (CEO) and DRS (Medical Director) are employees of HEB. WMM, WAC, and DRS own stock and options in HEB. LB, CWL, DLP, and BCS received clinical support for the conduct of AMP-516.
Download Provisional PDF Here
Aritcle Type: Research Article
Citation: Strayer DR, Stouch BC, Stevens SR, Bateman L, Lapp CW, et al. (2015) Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME): Characteristics of Responders to Rintatolimod. J Drug Res Dev 1(1): doi http://dx.doi.org/10.16966/2470-1009.103
Copyright: © 2015 Strayer DR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access