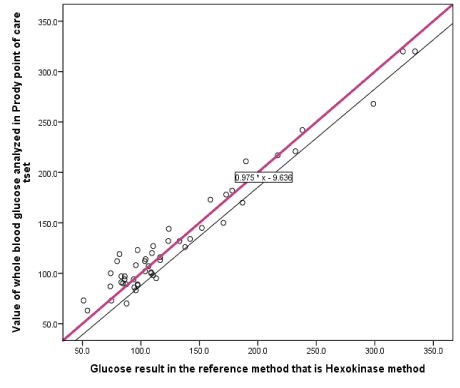
Figure 1: Linear regression graph of reference hexokinase glucose value versus prodigy glucose meter glucose value of DM patients at EPHI, Addis Abeba, Ethiopia, 2019.


Haymanot Tewabe1* Masiresha Tsigie2 Birhanu Haile1 Tewodros Zerihun1 Demirew Bikela1 Mistire Wolde1
1Department of Medical Laboratory Sciences, College of Health Science, Addis Ababa University, Ethiopia*Corresponding author: Haymanot Tewabe, Department of medical laboratory Sciences, College of Health Science, Addis Ababa University, Ethiopia, Tel: 923005284; E-mail: Haymanottewabe@gmail.com
Introduction: Point of Care Glucometer device (POCG) is the most commonly applied method to monitor Diabetes Mellitus (DM) worldwide. The aim of this study was to assess the accuracy of the prodigy glucose meter by using the hexokinase method as a reference.
Methods: A prospective cross-sectional study was done on a total of 52 randomly selected DM suspected patients from March 22 to April 13, 2019, in the Department of Medical Laboratory, Addis Ababa University, Ethiopia. Glucose value was determined by using prodigy glucose meter and the hexokinase method and data were entered and analyzed using SPSS software. The minimum and maximum accuracy of the prodigy glucometer were determined based on ISO 15197:2003 and ISO 15197:2013 criteria.
Results: The average serum glucose value measured by prodigy glucose meter and reference hexokinase methods was 132.52 mg/dl and 130.94 mg/ dl respectively. There was a statistically significant difference between the two methods (p-value <0.001). The bias of prodigy from the comparative method was 1.56 with a strong positive relationship (R=0.975; >80%). Besides, the prodigy glucose meter did not fulfill the minimum accuracy requirements of ISO 15197:2003 and ISO15197:2013.
Conclusion: The present study demonstrated that prodigy glucose meter readings are relatively higher than the reference method. In addition, the prodigy glucose meter did not fulfill the expected standards set by ISO. Thus DM patients need to evaluate the device before using it in standard laboratories. Furthermore, large scale studies including both DM and healthy study participants should be undertaken.
Prodigy glucometer; Diabetes Mellitus; Point of care devices
CI: Confidence Interval; DM: Diabetes Mellitus; DMLS: Department of Medical Laboratory; EPHI: Ethiopian Public Health Institute; GOD: Glucose Oxidase Method; ISO: International Organization for Standardization; POCG: Point of Care Glucometer; SPSS: Statistical Package for Social Sciences; SD: Standard Deviation; TAT: Turnaround Time
Diabetes Mellitus (DM) is a disease that requires continuing control and health education with enough support to prevent and to reduce the disease-associated complications. DM is complex diseases that need more advanced control and risk reduction in addition to serum/ plasma glucose level control [1,2]. The measurement of glucose level in blood is the chosen method for controlling and preventing DM [3]. Self-monitoring blood glucose levels by using a different Point of Care Device (POCG) devices have an important role in the control of DM and in the reduction of risk of serious secondary clinical complications associated with the diseases [1].
Currently, in the world, there are many different blood glucose concentration measurement techniques that have their own principles and method of measurement. This method and principle difference, as well as procedures how to use them, may lead to the variation of blood glucose results, as many studies indicate [4].
A Prospective study done in the University of Lagos, Nigeria, in 2017; to compare glucose oxidase method with three point-of-care measuring devices on a total of 150 confirmed diabetic patients indicates that; there were significant differences in the mean value of glucose using glucose oxidase method when compared with the point-of-care testing devices (p<0.05) [5]. On the other hand, another study done in Iraq at Rezgary and howler teaching hospitals ErbilIraq on admitting female patients by cross-sectional method in 2017; shows that 94% of the samples measured by POCG method were within ± 20% of the laboratory values of the value determined with the hexokinase method which supports the idea that point of care glucose testing can be used as a part of diagnostic process of diabetes mellitus [6]. Differences in blood glucose results for patients due to method variation are the main difficulty of clinical diagnosis and for DM control. So that, POCG device which has good accuracy as that of the reference method is needed for early detection, treatment and follow up the purpose of DM [4]. POCG testing has an advantage over reference hexokinase method by different mechanisms like the reduction of turnaround time, reduced pre-analytic and post-analytic testing errors, rapid data availability, self-contained and user-friendly instruments, reduction of patient length of stay in the laboratory, and use of small sample volume for a given test [7-9].
The accuracy and reliability of the POCG glucose monitor depend on device methodology and other factors, like sample source, collection time, usage of the POC glucometer device and patient characteristics. There are also different blood parameters capable of influencing measurements by POCG like variations in pH, hematocrit, blood oxygen, and microcirculation therapy and the amount of fatty tissue in the whole blood during figure drop measurement [9]. These factors in combination or alone can significantly enhance blood glucose measurement accuracy with POC glucose monitoring devices. Since inaccurate result due to these factors causes a high risk to false therapeutic decisions. So Standardized and regular evaluation of blood glucose meters and test strips should be requested in order to ensure the accuracy and quality standards of POCG cited by ISO 15189:2003 and ISO 15189:2013 [10]. The use of glucose meters like Prodigy auto code glucometer for blood glucose monitoring in DM patients is increasing from time to time in Ethiopia. Therefore, the aim of this study was to assess the accuracy of the Prodigy Auto code glucose meter comparing with reference hexokinase method in an automated machine (Cobas 6000 c501 clinical chemistry machine, hexokinase method) among randomly selected diabetic suspected patients in Ethiopia Public Health Institute (EPHI), Ethiopia.
Prodigy auto code blood glucose monitoring system is one of the POCG which is used for only in vitro diagnosis of blood glucose concentration the advantage of providing economical wastage minimization with advanced technology and with short turnaround time additional with its highest quality. A very small amount of blood (1 microliter) taken from the finger or alternative sites. The test strips automatically draw the blood sample into the test strip and give the results in a few seconds. The electrochemical method of this POCG uses Glucose Oxidase (GOD) enzymes which specifically catalyzes glucose and reduce interferences. This makes it an improved specific method of determination of blood glucose than other glucose meters which did not have high specificity to glucose [6].
This was a prospective cross-sectional study conducted on a total of 52 randomly selected diabetic mellitus voluntary individuals, in the Department of Medical Laboratory Sciences, Addis Ababa University, Ethiopia and Ethiopian Public Health Institute (EPHI) in the period between March 22 to April 13, 2019. The comparison was done at the EPHI clinical chemistry laboratory (which is an accredited laboratory by Ethiopian National Accreditation Agency and recognized by ISO). All volunteer patients visited EPHI during the study period were with all types of diabetic’s complications, including type I DM, type II DM and GDM.
Information regarding demographic characters like age, gender, medication, clinical history and type of diabetes were gathered on a pr-designed structured questioner. Blood samples were collected after overnight fasting (8-12 hours) of study participants, from the medial cubital vein and from the capillary of finger for the reference hexokinase method and Prodigy Auto Code blood glucose meter glucose measurement, respectively. Blood samples were collected after cleaning the collection site by, 70% of alcohol.
Blood glucose levels were measured by using Prodigy Auto Code blood glucose meter from finger prick whole blood for each of 52 participants. A drop of whole blood was applied to the electrode/strip and the reading was noted on the digital window. Simultaneously serum glucose level collected by Serum Separated Test tube (SST) was measured by using the hexokinase method in the fully automated machine (Cobas 6000 c501 clinical chemistry machine, hexokinase method). A 3-5 ml of blood was drawn from the median cubital vein into SST tube and transported to the clinical chemistry laboratory section. Serum was separated by centrifugation, at a speed of three 2500 RPM for five minutes with a relative centrifugal force of 1500 and finally analyzed on the automated analyzer (Cobas 6000 c501 clinical chemistry machine) by hexokinase method. Then capillary blood glucometer values measured by prodigy glucometer were compared with the hexokinase method which is the reference and the gold standard one. All measurements were done according to the manufacturers’ instructions by minimizing all sources of errors in both cases.
The collected data were entered and analyzed using the Statistical Package for Social Sciences (SPSS) version 21.00.0 (IBM Statistics, USA). The Bland Altman analysis was used to see the agreement of the Prodigy Auto Code blood glucose meter with reference Hexokinase method. P-value is calculated by ANOVA and used to detect the presence of an association between the two variables. The correlation coefficient and regression line were used to observe the degree of association of the prodigy blood glucose meter with the reference hexokinase method.
The prodigy glucose method was done in the EPHI reception section during blood sample collection and the reference hexokinase method was done on a Cobas 6000 c501 clinical chemistry machine at the EPHI clinical chemistry laboratory. Quality control is analysis every morning both for the hexokinase method and for the Prodigy blood glucose meter before starting test analysis by using specific control provided by the manufacturer. Accuracy was evaluated using the International Organization for Standardization (ISO) 15197:2003 and ISO 15197:2013 requirements by calculating the percentage of prodigy glucometer results falling within 5%, ± 10%, ± 15% and ± 20% of the reference value of hexokinase for glucose concentrations ≥ 75 mg/dl and ≥ 100 mg/dl and within ≥ 5, ± 10, ± 15 and ± 20 mg/dl of the reference value hexokinase method for glucose concentrations <75 mg/dl and <100mg/dl. The minimum acceptable accuracy for results produced by prodigy glucose meter according to ISO 15197:2003, is: ≥ 95% of the individual glucose results in prodigy glucometer shall fall within ± 15 mg/dl of the results of the manufacturer’s measurement procedure at glucose concentrations <75 mg/dl and within ± 20% at glucose concentrations ≥ 75 mg/dl and according to ISO 15197:2013, is: ≥ 95% of the individual glucose results shall fall within ± 15 mg/dl of the results of the manufacturer’s measurement procedure at glucose concentrations <100 mg/dl and within ± 15 at glucose concentrations ≥ 100 mg/dl [11-14]. In addition, the Bland-Altman plot was used to estimate the difference (bias) limits containing 95% of data because normally distributed differences were needed.
The study protocol was ethically approved by the Department of Medical Laboratory Science (DMLS), College of health science, Addis Ababa University and Ethiopian Public Health Institute (EPHI). Data were collected after consent was obtained from the study participants. To keep confidentiality, non-identifier codes which are not known by the unauthorized person were used.
In our study, a total of 52 volunteer DM patients participated, and of which 27 (51.9%) were males and the rest 25 (48.1%) were females. The average age of participants was 44.92 years (with minimum and maximum age 18 and 79 years; Median age=45 years). The minimum glucose concentration measurements in the prodigy glucometer and hexokinase method were 63 mg/dl and 50.9 mg/dl with a maximum reading of 320 mg/dl and 334.3 mg/dl respectively. The mean serum glucose value measured by the reference hexokinase method was 130.94 mg/dl and the mean capillary blood glucose value measured by prodigy Auto Code glucose meter was 132.52 mg/dl. In our study finding, there was a statistically significant difference between the means of prodigy glucose meter and reference glucose hexokinase method glucose value (p-value <0.001). The bias of the prodigy glucometer glucose valve from the reference hexokinase method was 1.56 and the strength of association (correlation coefficient) between the two methods was 0.975 which indicates that was a strong positive relationship between the two methods as shown in table 1.
| Parameters | Prodigy Method | Hexokinase Method | |
| Percentile | 25 | 94.00 | 87.48 |
| 50 | 112.50 | 108.65 | |
| 75 | 148.75 | 157.50 | |
| Minimum | 63.00 mg/dl | 50.90 mg/dl | |
| Maximum | 320.00 mg/dl | 334.30 mg/dl | |
| Mean | 132.52 | 130.94 | |
| Standard deviation | 59.63 | 64.89 | |
| Difference b/n means(bias) | 1.56 | ||
| P-valve | 0.001 | ||
| 95% confidence interval | -2.5746 to 5.7246 | ||
| Correlation coefficient | 0.975 | ||
Table 1: Comparison of glucose value statistics between the prodigy glucometer glucose level and the reference hexokinase method of patients at EPHI, Addis Ababa, Ethiopia, 2019.
The mean difference between the two methods was not statistically associated with sex and age (p-value of 0.355, 0.353 respectively) and also no statistically significant between the reference hexokinase method and time of sample collection (p=0.053 with an average time interval of 3 hours and SD=1.5). However, the mean bias showed a statistically significant association with glucose value with both methods. The mean bias increases as glucose value increase in hexokinase method methods (p-value <0.001) and no association with the prodigy blood glucose method (P-valve=0.082) (Table 2).
| Characteristics | Mean | P-valve | |||
| Age( years) | 44.92 | 0.980 | |||
| Characteristics of sex | Number | Percentage | P-valve | ||
| Gender | Male | 27 |
51.9 | 0.345 | |
| Female | 25 |
48.1 | |||
| Characteristics of blood glucose | Mean | P-valve | |||
| Glucose reading result using Prodigy glucometer in (mg/dl) | 132.52 | <0.001 | |||
| Glucose reading result in Hexokinase method in (mg/dl) | 130.94 | <0.001 | |||
| Difference in glucose level (mg/dl) Glucometer vs hexokinase method | 1.56 | 0.100 | |||
Table 2: Percentage and Mean Distribution of demographic and blood glucose Characteristics and the association between the mean bias of glucose with other variables among DM patients at EPHI, Addis Ababa, Ethiopia, 2019.
The slope of the regression line for reference hexokinase method versus prodigy glucose meter glucose values was 0.975 with a negative intercept of 9.636 mg/dl. Under the simultaneous equation, the Y=X and Y=0.975X-9.636 graphs do not meet at a point and this indicates that it is statistically significant in the bias difference of the two methods (Figure 1).

Figure 1: Linear regression graph of reference hexokinase glucose value versus prodigy glucose meter glucose value of DM patients at EPHI, Addis Abeba, Ethiopia, 2019.
The Bland-Altman plot showed that most of the difference (bias) glucose values between prodigy glucose meter and reference hexokinase methods lay within the bias ± 1.96 SD (95% CI). The 95% limit of agreement was -27.629 to 30.779 (Figure 2).
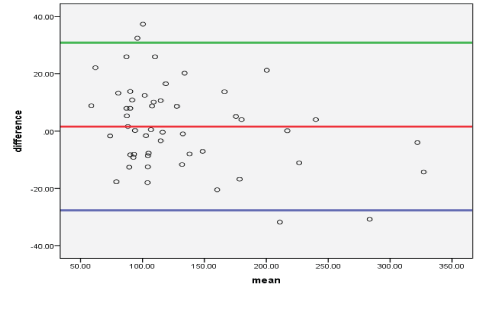
Figure 2: The bias plot (Bland-Altman plot) of DM patients’ glucose value between reference hexokinase glucose values versus prodigy glucose meter glucose value of DM patients at EPHI, Addis Abeba, Ethiopia, 2019.
The percentage of prodigy blood glucose values deviation from the hexokinase reference method is shown below in table 3. ISO 15197 has its own requirements for different measurements and the minimum requirement according to ISO 15197:2003 is; ≥ 95% of the individual glucose results shall fall within ± 15 mg/dl of the results of the prodigy glucometer measurement at glucose concentrations <75 mg/dl and within ± 20% at glucose concentrations ≥ 75 mg/dl and according to ISO 15197:2013 is ≥ 95% of the individual glucose results shall fall within ± 15 mg/dl of the results of the prodigy glucometer measurement procedure at glucose concentrations <100 mg/dl and ≥ 100 mg/dl [11-14].
| Cut point of the reference Method | Percentage of prodigy glucose levels within the reference method intervals | ||||
| Within ± 5% (5 mg/dl) |
Within ± 10% (10 mg/dl) |
Within ± 15% (15 mg/dl) |
Within ± 20% (20 mg/dl) |
Beyond ± 20% (20mg/dl) | |
| <75 mg/dl | 2/4 (50%) | 4/4 (100%) | 4/4 (100%) | 4/4 (100%) | 0/4 (0%) |
| ≥ 75 mg/dl | 1/48 (2.13%) | 5/48 (10.64%) | 9/48 (19.15%) | 11/48 (23.40%) | 36/48 (76.60%) |
| Over all | 3/52 (5.77%) | 9/52 (17.31%) | 13/52 (25.00%) | 15/52 (28.85%) | 36/52 (69.23%) |
| <100 mg/dl | 6/18 (33.33%) | 10/18 (55.56%) | 17/18 (94.44%) | 17/18 (94.44%) | 1/18 (5.56%) |
| ≥ 100 mg/dl | 0/34 (0.00%) | 1/34 (2.94%) | 4/34 (11.76%) | 5/34 (14.72%) | 29/34 (85.29%) |
| Over all | 7/52 (13.46%) | 11/52 (21.54%) | 21/52 (40.38%) | 22/52 (42.31%) | 30/52 (57.69%) |
Table 3: Percentage of prodigy glucose meter results falling within various intervals ISO requirements within the reference glucose hexokinase value of DM patients at Ethiopia Public Health Institute (EPHI), Ethiopia, 2019.
But in our study, the result did not fall within the minimum requirement in both cases especially in the case of glucose concentrations ≥ 100 mg/dl and ≥ 75 mg/dl. This indicated that the prodigy did not fulfill the minimum requirements set by ISO 15197:2003 and ISO 15197:2013.
According to our study finding, the minimum and maximum glucose concentration measured by prodigy glucose meter and reference hexokinase methods were 63 mg/dl and 3,320 mg/dl and 50.9 mg/dl and 334.4 mg/dl with mean 130.94 mg/dl and 132.52 mg/ dl respectively (table 1). In our study, the result did not fall within the minimum requirement in both cases especially in the case of glucose concentrations ≥ 100 mg/dl and ≥ 75 mg/dl which is in line with the finding of the prospective study done in the University of Lagos, Nigeria, in 2017; to compare glucose oxidase method with three pointof-care measuring devices on a total of 150 confirmed diabetic patients and in opposite to the finding of the study done in Iraq at Rezgary and howler teaching hospitals Erbil-Iraq on admitting female patients by cross-sectional method in 2017; which shows that 94% of the samples measured by POCG method were within ± 20% of the laboratory values of the value determined with the hexokinase method which supports the idea that point of care glucose testing can be used as a part of diagnostic process of diabetes mellitus [6].
The mean difference (bias) between the two methods was 1.56. The mean difference (bias) between the two methods was not statistically significant with associating with sex and age (p-value of 0.355 and 0.053 respectively) and also no statistically significant between the reference hexokinase method and time interval of the sample between collection time and analysis time (p-value 0.053). However, the mean bias showed a statistically significant association with both reference hexokinase method and prodigy glucose values (p-value <0.001 and <0.001, respectively) (Table 2). The bias between the two methods increases as the concentration of glucose increases.
This study showed that; 4/4(100%) of the prodigy glucose measurement results fall within ± 15 mg/dl of the results of the reference glucose oxidase method at glucose concentrations <75 mg/dl and 11/48(23.40%) of the prodigy glucose measurement results fall within ± 20% mg/dl of the results of the reference glucose oxidase method at glucose concentrations ≥ 75 mg/dl. In addition, 17/18(94.44%) and 4/34(11.76%) of the prodigy glucose measurement results fall within ± 15 mg/ dl of the results of the reference glucose hexokinase method at glucose concentrations <100 mg/dl and ≥ 100 mg/dl, respectively. However, according to ISO 15197 criteria ≥ 95% the prodigy glucose measurement results should fall within the above reference glucose value intervals [11-14]. Therefore, the prodigy glucose meter did not fulfill the minimum accuracy requirements of ISO 15197.
POCG devices are a better alternate solution in developing countries like Ethiopia which has a shortage of skilled manpower, electricity, and other obstacles besides their short TAT, price and traceability. Variety of POCG device is available on the market for blood glucose measurement. Even if; different conditions like the designed working environment including temperature and humidity, the sensitivities and specificities of the machines, as well as procedures differ from device to device. Therefore, before the introduction of POCG devices for personal use and laboratory services, they should be evaluated by comparing with the reference methods, and standards set by accredited quality standardizing organizations such as the ISO. And also programmed quality control must be performed based on the manufacturer instruction.
Our study was done to assess the accuracy of the prodigy glucometer glucose result in relative with the standard reference hexokinase method using Cobas 6000 c501 clinical chemistry machine in EPHI clinical chemistry laboratory, Ethiopia. The findings showed that prodigy glucometer glucose performance did not fulfill the minimum accuracy requirements cited both by ISO 15197:2003 and 15197:2013 standards criteria.
Even though; there is a strong positive correlation between prodigy glucose meter and the reference hexokinase methods of glucose level (R=0.975); there was statistically significant bias difference between the two methods in determining blood glucose concentrations with bias difference of prodigy glucose meter glucose value averagely by 1.56 from reference hexokinase method. Therefore, DM individuals should evaluate the performance of prodigy Glucometer at standard reference laboratories before using them.
Even if our study finding shows that; the glucose measurement by using prodigy glucometer did not fulfill the minimum requirement of both ISO 2003 and 2013 standards; to generalize this conclusion; further study should be undertaken with increasing the sample size depends on age and sex, with consideration of types of diabetes, as well including both health and DM study participants, by using different prodigy point of care devices which are easy to use and mostly available in the country. Additional with this recommendation; classification of diabetic groups during the study as a control group and non-control group is another basic thing to set generalized recommendation about the finding of the study. Also, we recommended that it was better if it is done by classifying diabetic groups into hypoglycemic, hyperglycemia and normoglycemic individuals to see the accuracy of a prodigy in low and normal levels of blood glucose in addition to the high blood glucose level in diabetes mellitus patients.
During the study period of this research, there was no type of potential conflict interest with respect to the research, authorship, and/ or publication.
This study was supported by the Department of Medical Laboratory, College of Health Sciences, Addis Ababa University, Ethiopia by granting a POCG device prodigy glucometer with enough specific strips.
The authors acknowledge diabetes mellitus patients voluntarily participate in the study. In addition, our acknowledgment goes to Ethiopian Public Health Institutes (EPHI), for their permissions to use the Clinical chemistry laboratory; and the Department of Medical Laboratory Sciences Addis Ababa University (DMLS, AAU) volunteer donation of Prodigy glucometer with devices. Finally, acknowledge all EPHI and DMLS-AAU staff encouraged us during the study periods.
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Tewabe H, Tsigie M, Haile B, Zerihun T, Bikela D, et al. (2019) Evaluate Performance of Prodigy Glucose Meter versus Reference Hexokinase Method in Addis Ababa, Ethiopia. J Diab Res Ther 5(2): dx.doi.org/10.16966/2380-5544.144
Copyright: © 2019 Tewabe H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access