Abstract
Background: Published clinical trials of SGLT2 inhibitors have typically recruited participants with modest elevations of HbA1c. Our aim was
to evaluate the efficacy of dapagliflozin in patients with uncontrolled glycaemia in a routine clinic setting where patients are taking a range of
other therapies.
Methods: A prospective observational evaluation over six months. Dapagliflozin was added to concomitant medications including insulin and
glucagon like peptide-1 (GLP-1) agonists. Clinical outcomes were examined in patients with a baseline HbA1c ≥ 9.5% (80 mmol/mol) and an
HbA1c<9.5% (80 mmol/mol).
Results: Data was available on 166 patients (65 HbA1c<9.5%; 101 HbA1c ≥ 9.5%). All showed significant reductions in HbA1c, body weight,
blood pressure and insulin dose. Those with an HbA1c ≥ 9.5% showed greater reduction in HbA1c (10.8% to 9.3% [94.8 to 78.3 mmol/mol])
than those with an HbA1c<9.5% (8.6 to 8.1% [70.6 to 65.1 mmol/mol]), P<0.001. Those with HbA1c<9.5% had a greater mean weight reduction
(104.0 to 100.3 kg v 104.3 to 102.5 kg, P<0.001), which was associated with greater reduction in insulin dose (102 to 83 U/day v 91 to 84 U/day,
P<0.001). Reductions in all concomitant medications occurred.
Conclusion: Within our routine practice, dapagliflozin was associated with a greater reduction in HbA1c in patients with worse glycaemic
control.
Keywords
Dapagliflozin; HBA1c; Poor glycaemic control
Introduction
The sodium-glucose co-transporter 2 (SGLT2) inhibitors are a novel
class of oral anti-diabetic medication, which inhibit renal glucose
reabsorption. In a healthy individual, approximately 180 g of glucose is
filtered and reabsorbed by the kidney each day. Approximately 90% of
glucose reabsorption is mediated by SGLT2, located in the proximal
tubule of the kidney and the remaining 10% by the sodium-glucose cotransporter
1 (SGLT1) [1]. In a person with poorly controlled diabetes,
SGLT2 is up-regulated, leading to increased renal glucose reabsorption
which contributes to chronic hyperglycaemia [2,3]. SGLT2 inhibitors
lead to increased urinary glucose excretion with resulting lower blood
glucose concentration and net calorie loss [4]. This effect is dependent
on glomerular filtration rate and plasma glucose concentration but
is independent of insulin [4]. Dapagliflozin, the first selective SGLT2
inhibitor, gained approval by the European Medicines Agency in
November 2012. In the UK, dapagliflozin is recommended by the National
Institute of Heath and Clinical Excellence (NICE) as monotherapy or
in combination with other anti-diabetic medications including insulin
in Type 2 diabetes [5]. Pre-registration randomised control trials have
demonstrated that dapagliflozin improves glycaemic control and reduces
body weight and blood pressure when used as monotherapy [6], with
other oral hypoglycaemic agents [7-10] or insulin [11]. Clinical trial
protocols have typically recruited participants with a modest elevation of
HbA1c such that the mean baseline HbA1c is in the range of 7.7%-8.57%
[6,8-11]. These trial participants do not reflect the full range of glycaemic
control seen in the diabetes population. There is little published experience
relating to the effectiveness of dapagliflozin in poorly controlled patients. A
preliminary analysis using subgroup data from five phase 3 trials (n=139)
suggests that dapagliflozin is associated with greater glucose reduction in
participants with baseline HbA1c ≥ 9% [12]. The greatest effect was seen
in treatment naïve patients with a short duration of diabetes. Our aim
was to investigate the efficacy of dapagliflozin in patients with the worst
glycaemic control in a routine clinical setting.
Methods
Subjects
We conducted a prospective observational audit in two secondary
care hospital diabetes clinics. Dapagliflozin was added to concomitant
diabetes medications including insulin and glucagon like peptide-1 (GLP-
1) receptor agonists as part of routine physician-led care. Consecutive
patients with type 2 diabetes initiated on dapagliflozin from October
2013 to December 2014 were included if they had attended for follow-up
assessment. Data were collected on patient demographics, the duration of
diabetes, concomitant anti-diabetic medications, body weight, body mass
index (BMI), blood pressure and HbA1c before and after dapagliflozin
treatment. We compared the effects of dapagliflozin in patients with poor
control using an HbA1c value ≥ 80 mmol/mol (9.5%) to define patients
with the poorest glycaemic control. This is a level above which physicians
would usually be considering insulin therapy rather than additional oral
hypoglycaemic agents. The American Diabetes Association (ADA) and
in the UK the National Institute for Health and Care Excellence (NICE)
recommend to consider insulin therapy if HbA1c>9% [13,14].
Statistical analysis
Statistical analysis was performed using SPSS (version 22, SPSS
Inc., Chicago). Results for continuous variables are presented as mean
and standard deviation. Baseline parameters in the two groups were
compared by t-test test for continuous variables or chi-square test
for categorical variables. The effects of dapagliflozin in patients with
baseline HbA1c ≥ 9.5% (80 mmol/mol) were compared with those with
baseline HbA1c<9.5% (80 mmol/mol) using repeated measures analysis
of variance. Change in each group over time was tested separately if the
interaction was significant. A P-value ≤ 0.05 was considered statistically
significant.
Ethical consideration
This study was designed as a clinical audit of routine clinical practice to
improve patient management. Therefore, this study did not require ethical
committee review.
Results
Results
We identified 187 patients who had been initiated on dapagliflozin
and attended ≥ 1 clinic follow-up visit. Twenty-one patients discontinued
dapagliflozin. Data is presented on the remaining 166 patients. The
reasons for discontinuation included candida infection (3 patients),
urinary tract infection (1), polyuria/polydipsia (2), pregnancy (1),
myocardial infarction (1), a reduction in estimated glomerular filtration
rate (1), campylobacter infection (1), bariatric surgery (1) and nonspecific
reasons in the remainder.
Patient sample
At baseline, the mean age of the 166 patients was 57.8 ± 9.4 years and
duration of diabetes 11.1 ± 6.5 years. Most (99%) were white Caucasian.
One hundred and eleven (64%) were male. The characteristics of the two
groups (HbA1c ≥ 9.5% or <9.5%) at initiation of dapagliflozin are shown
in table 1. There were no significant differences in baseline parameters
between the groups and the baseline eGFR was >60 ml/min/1.73m2
(by
MDRD equation) in all. The duration of treatment with dapagliflozin was
6.3 ± 2.6 months.
Efficacy in the whole group
In all the patients grouped together, body weight reduced from 104.2
± 18.2 to 101.6 ± 18.3 kg (P<0.001), BMI from 35.5 ± 5.9 to 34.5 ± 5.9 kg/
m2 (P<0.001), HbA1c from 9.95 ± 1.42 (85.5 ± 12.2 mmol/mol) to 8.84 ±
1.49% (73.3 ± 12.4 mmol/mol), P<0.001 with 21% of patients receiving
dapagliflozin achieving a HbA1c ≤ 7.5% (58 mmol/mol). Mean systolic
blood pressure (SBP) decreased from 138.7 ± 17.7 to 134.4 ± 16.9 mmHg
(P=0.002). Mean diastolic blood pressure (DBP) decreased from 78.7 ±
11.5 to 75.7 ± 9.8 mmHg (P=0.001).
Effect of dapagliflozin therapy on concomitant medications
One hundred and eleven patients (67%) were receiving insulin
therapy. Total daily insulin requirement decreased from 95.4 ± 55.5
to 83.4 ± 44.8 units (P<0.001). Forty-two patients (25%) were treated
with GLP-1 receptor agonists. Seven of the 42 were able to discontinue
GLP-1 agonist therapy. The number of patients taking a sulphonylurea
decreased from 49 to 39; DPP-4 inhibitor treatment from 19 patients
to 11; and pioglitazone from 7 to 2. There were 135 patients taking
metformin at baseline and 134 at follow-up. The mean dose of
metformin did not change (Table 1).
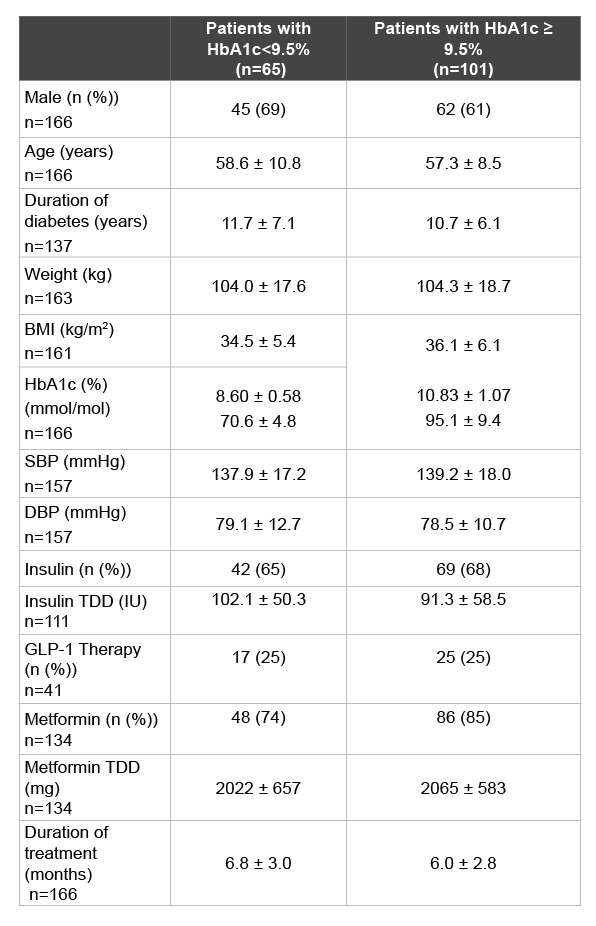
Table 1: Patient characteristics grouped by degree of glycaemic control at
initiation of dapagliflozin
BMI: Body mass index; SBP: Systolic blood pressure; DBP: Diastolic blood
pressure; TDD: TOTAL daily dose; GLP1: Glucagon like peptide 1.
None of the parameters show significant difference between the groups
(except HbA1c)
Efficacy in patients with baseline HbA1c ≥ 9.5% (80 mmol/mol)
versus those with baseline HbA1c <9.5% (80 mmol/mol)
At baseline the characteristics of both groups were similar (Table 1).
Mean changes from baseline in HbA1c, body weight, blood pressure
and daily insulin requirement are shown in table 2. In the group with a
HbA1c ≥ 9.5% the mean HbA1c decreased from 10.83% (95.1 mmol/
mol) to 9.30% (78.3 mmol/mol) whereas in those with a HbA1c<9.5%
(80 mmol/mol), the mean HbA1c decreased from 8.60% (70.6 mmol/
mol) to 8.13% (65.4 mmol/mol). The ANOVA showed an interaction
(P<0.001) indicating significantly greater response in patients with
poorest glycaemic control. Fifty-four patients had a clinically insignificant
reduction in HbA1c (<0.5%). These consisted of 33 (33 of 65:51%) in the
group with HbA1c<9.5% and 21 (21 of 101: 21%) in the group with an
HBA1c ≥ 9.5%.
With respect to body weight the reverse association was observed.
Weight decreased from 104.3 to 102.5 kg in the HBA1c ≥ 9.5% group
compared to 104.0 to 100.3 kg in the group with HbA1c<9.5% (interaction
P=0.001). As expected the same association was observed for BMI with a
decrease from 36.1 to 35.3 in the HBA1c ≥ 9.5% compared to 34.5 to 33.1
in the group with a HbA1c<9.5% (interaction P=0.028). The reductions
in SBP and DBP were similar between the two groups (interactions not
significant). The insulin total daily dose decreased from 91 to 84 U/day in
the HBA1c ≥ 9.5% group but there was a greater reduction from 102 to 83
U/day in the HBA1c<9.5% group (interaction: P=0.017).
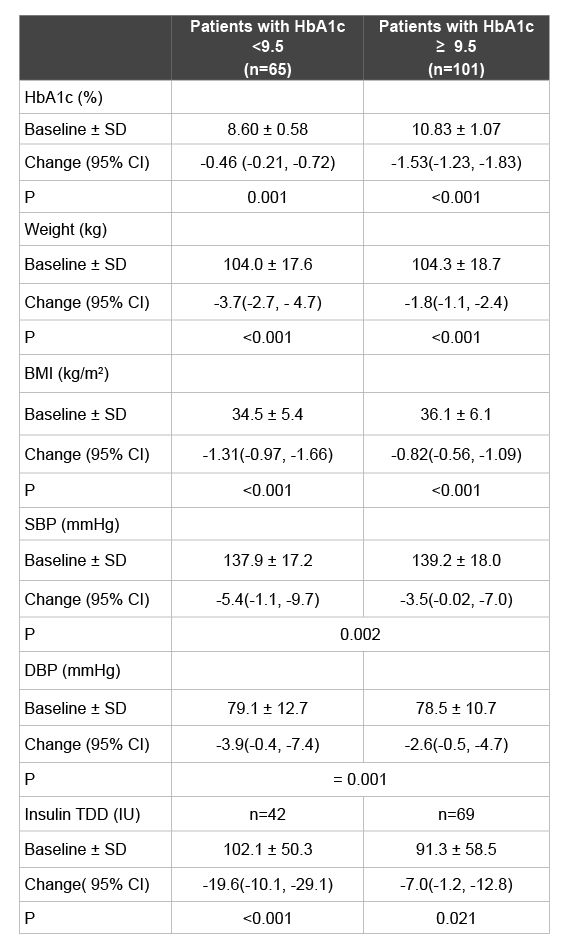
Table 2: Mean changes in efficacy parameters in those with baseline
HbA1c<9.5% (80 mmol/mol) & those with baseline HbA1c ≥ 9.5% (80
mmol/mol)
BMI: Body mass index; SBP: Systolic blood pressure.
The changes in HbA1c, body weight and BMI with dapagliflozin were
different between the two glycaemic control groups (interactions significant)
but not in the case of SBP and DBP where similar reductions were seen.
Discussion
Previous phase 3 clinical trials have shown that dapagliflozin is
effective in reducing HbA1c as monotherapy (-0.89%) [6]; as add-on to
metformin (-0.78%) [10]; as triple therapy with add-on to metformin and
sulphonylurea (-0.69%) [9]; and as add-on to insulin (-0.96%) [11]. In our
routine clinical evaluation we observed a greater mean reduction of 1.11%
(12 mmol/mol) (P<0.001) for the whole group. Of note, our patient sample
had a higher baseline HbA1c of 9.95 ± 1.42% (85.5 ± 12.2 mmol/mol)
compared to those recruited into published phase 3 clinical trials with
dapagliflozin. We observed that the efficacy of dapagliflozin in reducing
HbA1c was significantly greater in those patients with higher baseline
HbA1c ≥ 9.5%. This is consistent with subgroup analysis from trials
[6,9,12] and data from clinical practice [15]. The efficacy of dapagliflozin
on glucose control appears to be associated with baseline blood glucose
levels and renal function [4]. Thus, unlike other oral agents, dapagliflozin
is an option for the treatment of more poorly controlled patients and may
be an alternative to starting or increasing insulin therapy.
Twenty-one patients with a HbA1c ≥ 9.5% showed no clinically
significant effect with a reduction in HbA1c of less than 0.5%. It is possible
these patients were non compliant with therapy, particularly since SGLT2
inhibitors are not without unwanted effects. Alternatively, perhaps these
patients did not benefit from the reduction in glucose load that the
increased glycosuria achieves, and therefore were truly insulin dependent,
a judgement that is often difficult to make in the clinic. These results may be
related to the design of this observational study and associated limitations
when compared to a randomised, placebo-controlled prospective study.
Out patients had a relatively long duration of recognised diabetes (11
years), which may be associated with a greater chance of beta-cell
dysfunction. Nevertheless, it does seem that the majority of our poorly
controlled type 2 patients need a reduction in glucose load to achieve
better HbA1c, and this is achieved by SGLT2 inhibition. The response
to SGLT2 therapy may assist in the identification of genuinely insulin
dependent type 2 diabetic patients.
SGLT2 inhibitors generate a net loss of 200-300 kilocalories per day
as urinary glucose and have produced weight loss of between 2.1-3.2
kg in clinical trials [6,10,11,16]. In addition, the osmotic diuresis and
natriuretic effect of SGLT2 inhibition has been associated with a clinically
significant reduction in blood pressure. We observed similar significant
reductions in weight, BMI and blood pressure. Greater reduction in body
weight and BMI occurred in the lower HbA1c group and was associated
with a greater reduction in insulin dose.
A previous clinic-based analysis has also shown that dapagliflozin
is associated with a reduction in the need for concomitant diabetes
medication [15]. Within our study, there were no significant changes in
metformin total daily dose, but 20% of patients treated with a sulfonylurea
and 17% of those treated with a GLP-1 agonist were able to discontinue
these medications. The total daily insulin dose decreased by 11.7 units/
day. Thus in routine clinical practice, significant reductions in HbA1c is
achieved despite reductions in other diabetes medications. A reduction in
daily insulin dose is likely to make it easier for obese patients to lose weight.
Reductions in weight and blood pressure may translate into improved
long-term outcome. These changes and reductions in medications are well
appreciated by patients and have additional cost benefits. We observed a
low prevalence of reported genital and urinary tract infection, leading to
discontinuation of therapy. Phase 3 clinical trials have reported variable
rates from <1% to 8-9% [17]. It seems likely that poorly controlled patients
will have significant glycosuria prior to SGLT2 treatment such that this
therapy will make little difference in this group.
In summary, when used in routine clinical practice, dapagliflozin has
a greater efficacy when used in patients with a HbA1c ≥ 9.5%. This has
not been demonstrated well in the published phase 3 trials available to
date. We also observed significant reductions in concomitant medicine
use including insulin and GLP-1 agonists with a low discontinuation rate
of treatment.
Acknowledgements
TM and JNH wrote the manuscript and researched data AM and CM
researched data. JWS, DEP and AND reviewed and edited the manuscript.
JNH is the guarantor for the data and manuscript. JWS also contributed to
data preparation. There was no funding associated with this study.
Disclosures
The authors declare no conflict of interest, nor disclosures associated with
this manuscript. This study was conceived and conducted independently
of the companies that manufacture and market dapagliflozin.
Article Information
Article Type: Review Article
Citation: Min T, Mallipedhi A, MacIver C, Price
DE, Dixon AN, et al. (2016) Clinical Evaluation of
Dapagliflozin in Patients with Poor Glycaemic Control
in the Routine Clinical Setting. J Dia Res Ther 2(2):
doi http://dx.doi.org/10.16966/2380-5544.118
Copyright: © 2016 Min T, et al. This is an open
access article distributed under the terms of
the Creative Commons Attribution License,
which permits unrestricted use, distribution, and
reproduction in any medium, provided the original
author and source are credited.
Publication history:
Received date: 13 Jan 2016
Accepted date: 15
Mar 2016
Published date: 21 Mar 2016



