Abstract
The acceleration in the rate of chronic diseases that involve insulin resistance has become of global concern. The rate of the most prevalent
chronic disease such as cardiovascular disease is linked to the metabolic syndrome, non alcoholic fatty liver disease (NAFLD) and other chronic
diseases that include obesity, diabetes and neurodegenerative diseases. The gene-environment interaction in Western countries indicates
that with urbanization access to food and its content may lead to induction of epigenetic alterations and identify the gene Sirtuin 1 (Sirt 1)
to be responsible for the increased risk for insulin resistance and NAFLD relevant to Type 1, Type 2 and Type 3 diabetes in these countries.
Nutrigenomics is linked to neuron and liver telomere maintenance, cell division and tissue growth and has become important with essential
nutrients that regulate Sirt 1 function important to prevent NAFLD in individuals with diabetes. Nutrigenomic diets, exercise, drugs and lifestyle
changes regulate Type 3 diabetes with neuron Sirt 1 transcriptional responses associated with DNA modifications that regulate brain insulin
resistance relevant to NAFLD and diabetes.
Keywords
Nutrigenomics; Obesity; Diabetes; Accelerated Aging; Endocrine; Chronic Diseases; Diet
Background
In Western countries and third world countries the global obesity
epidemic has been reported to effect at least 10% of the global population
with projected health care costs to obesity related medical expenses
reported to be 344 billion dollars to the year 2018 and may account for 21%
of health costs in the United States. The acceleration in the rate of chronic
diseases that involve insulin resistance has also become of global concern.
The rate of the most prevalent chronic disease such as cardiovascular
disease [1-4] is linked to the metabolic syndrome and non alcoholic fatty
liver disease (NAFLD) and environmental factors such as stress, anxiety
and depression are important to consider with the global increase in other
chronic diseases that include diabetes and neurodegenerative diseases.
In 2013 the world health organization (WHO 2013) indicated that the
number of global deaths (63%) included cardiovascular disease (48%),
cancer (21%) and chronic respiratory conditions (12%). Nutritional
and anti-aging therapies may prevent accelerated aging involved in
the global obesity epidemic that currently involves various endocrine
and chronic diseases in Western populations. Obesity and diabetes
are defined as endocrine disorders with the medical conditions such
as hyperinsulinemia involved with hormonal imbalance with various
inflammatory complications of organs such as the liver, brain, thyroid,
parathyroid, adrenal gland and pancreas [5,6]. Stress, fatigue, anxiety
and depression are closely linked to chronic diseases and the molecular
mechanisms that involve the apelinergic pathway have become important
to Type 3 diabetes and neuroendocrine disease with theinduction of
insulin resistance that lead to obesity and diabetes [7-12]. Metabolic
diseases such as cardiovascular disease and neurodegeneration are
associated with accelerated aging process and involve alteration in
blood lipids such as cholesterol and triglyceride with a decrease in high
density lipoprotein (HDL) [13-16]. Anxiety disorders effect mental
health and induce changes in the brain associated with plasma hormone
dysregulation and alterations in the biological clock especially in tissues
such as the liver, adipose tissue and brain.
The gene-environment interaction in Western countries indicates
that with urbanization [17] and access to food and its content may lead
to induction of epigenetic alterations that are associated with lipid and
glucose dyshomeostasis with increased risk for insulin resistance relevant
to Type 1, Type 2 and Type 3 diabetes and obesity in these countries.
Diets that contain various organic pollutants with overnutrition lead
to abnormal xenobiotic metabolism [18-22] with marked effects on
DNA strand breakage with cell apoptosis. High calorie diets, exercise
and lifestyle changes regulate transcriptional responses with DNA
modifications that include DNA methylation, histone tails, chromatin
and micro RNA (short ribonucleic acid gene regulators)that regulate DNA
expression and promote chronic disease susceptibility (Figure1). The
gene environment interaction now identify the nuclear receptor Sirtuin
1 (Sirt 1)that regulates appetite [23] to be involved in the induction of
insulin resistance and Type 3 diabetes and involve alterations in nuclear
receptors, micro RNA with chromatin remodelling [24] that are now
closely associated with obesity, diabetes and neurodegenerative disease.
Sirtuin 1 contributes to the Post-Transcriptional
Dysregulation in Type 1, Type 2 and Type 3 Diabetes
Nutritional research has concentrated on the identification of nutrient
sensing diets that provide regulation of histone deacetylases (HDAC)
that are involved in epigenetic control of gene expression that controls
metabolic and tissue glucose and cholesterol homeostasis [7,14,17].
Sirt 1 is a NAD+ dependent protein deacetylase and is involved in
the deacetylation of the nuclear receptors (Figure 1) with its critical
involvement in insulin resistance [25]. The anti-aging protein Sirt 1 and
cell senescence has been closely linked to telomere biology and global
DNA repair which provides mechanistic explanations for Sirt 1 functions
in the protection of DNA damage, and thus genomic stability [26,27] with
relevance to Type 1, Type 2 and Type 3 diabetes. Anti-aging strategies
that target telomere shortening and brain glucose dysfunction in obesity
and diabetes are of particular interest to biological aging since telomere
shortening has been associated as an early risk for dementia and Type 3
diabetes.
In calorie restriction regulation of the Sirt 1 gene involved in
lifespan and aging has now become essential therapy for glucose and
cholesterol maintenance with reversal of chronic diseases such as obesity,
diabetes and neurodegenerative diseases in global communities [24].
Considerable interest in chronic diseases of the central nervous system
that include neurodegenerative diseases such as Parkinson’s disease
(PD) and Alzheimer’s disease (AD) may provide a useful model for the
prevention and management of various chronic diseases. Risk factors
such as diet and lifestyle indicate that Sirt 1 dysregulation is important to
liver and metabolic health [24] and central to Type 3 diabetes since Sirt
1 is involved in neuron loss [28] associated with insulin resistance and
neurodegenerative disease.
In Figure 2 a summary is shown of the relevance of Sirt 1 in the genetic
regulation of diabetes. HLA class I, II genes and genes of the HLA region
of chromosome 6 have also been shown to be involved greatly with the
risk for Type 1 diabetes (HLA-DR, DQ and DP). In caucasian populations
contribution by DQA1and DQB1 haplotypes, linkages to DRB1*03 and
DRB1*04 haplotypes have been associated with Type 1 diabetes. Other
ethnic groups such as African, Americans, Japanese and Chinese specific
haplotypes have also been associated with Type 1 diabetes [29]. An interest
in global chronic diseases has identified Sirt 1 dysregulation to involve
Type 1 diabetes with nutritional regulation of Sirt 1 important to assist
with glucose homeostasis in these Type 1individuals. Sirt 1 regulation
of the MODY gene via transcription factors hepatocyte nuclear factor
1(HNF-1) and HNF-4 or HNF1/HNF4 complex [30-32] has been shown
with evidence of Sirt1/HNF-4genetic regulation of liver and pancreas in
Type 1 diabetes.
In Type 2 diabetes more than 150 genetic loci are associated with the
development of diabetes and 50 candidate genes have shown to play
a major part in the development of the disease and include genes such
as peroxisome proliferator-activated receptors, ATP-binding cassette
transporter sub-family C member 8, KATP mutations and Calpain 10 [29].
These genes are involved in pancreatic β cell function, insulin action and
glucose metabolism in metabolic conditions (Figure 2). In Type 2 diabetes
the relevance of stress to defective apelinergic pathways that involve the
pancreas, liver, kidney and brain have been identified [7] with severity
of diabetes associated with poor insulin actions and glucose regulation.
Sirt 1 plays an important role in the regulation of the apelinergic pathway
relevant to Type 2 diabetes with connections to brain insulin resistance
(stroke, dementia, AD) and Type 3 diabetes [7]. Individuals with Type
1 and Type 2 diabetes involve early and accelerated organ diseases of
the brain when associated with Type 3 diabetes and the liver with Sirt
1 repression closely involved with insulin resistance in these individuals.
A single gene effect associated with Sirt 1 repression versus multiple
diabetic genes effect may indicate either the interaction of the diabetic
individual is sensitive to gene-environments events that involve diet and
lifestyle changes. Cellular miRNA have become important since they may
be regulated by diet, drugs, xenobiotics, stress and anxiety (environment)
that may be relevant to the stress sensitive Sirt 1 [7]. Nutritional
intervention is now critical early in life to maintain normal Sirt 1/
fibroblast growth factor 1 regulation of circadian rhythm that maintain
brain-liver pathways and glucose homeostasis [32,33]. Sirt 1 regulation
targets various transcription factors peroxisome proliferator-activated
receptor-gamma co-activator (PGC-1 alpha), p53 tumour suppressor
protein, pregnane x receptor (PXR) to adapt gene expression to metabolic
activity, insulin resistance and inflammation [24]. Effects of feeding on Sirt
1and p53 interactions are involved in nuclear-mitochondria interactions,
mutations, cell death (apoptosis) or permanent cellular senescence [24]
with relevance to Sirt 1 deacetylation of p53 that determine DNA repair
critical for maintenance of cell glucose homeostasis [34,35].
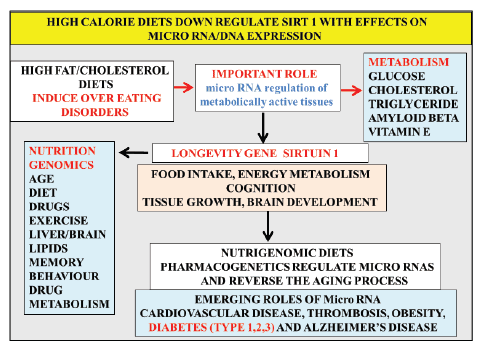
Figure 1: High calorie diets, stress, exercise and lifestyle changes regulate transcriptional responses with DNA modifications that regulate neuron
micro RNA and DNA expression and promote chronic diseases associated with obesity, diabetes (Type 1,2,3) and neurodegenerative disease. The
gene environment interaction indicates that the stress sensitive anti-aging gene Sirtuin 1 is downregulated with effects on drug therapy linked to the
induction of Type 3 diabetes.
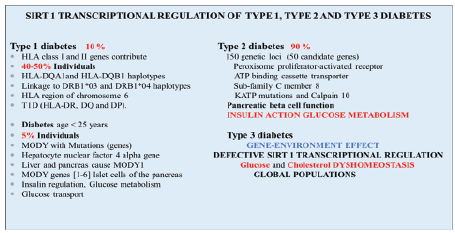
Figure 2: The genetic regulation of Type 1 and Type 2 diabetes is associated with the gene expression of Sirt 1 in global populations. A single
gene effect associated with the repression of the anti-aging gene Sirt 1 versus multiple diabetic genes may indicate the interaction of the diabetic
individual to gene-environments events such as unhealthy diets and stress that involves Sirt 1 dysregulation of glucose and cholesterol in the brain
and peripheral tissues.
Effects of p53 on gene regulators include micro RNA (miRNAs)
[36,37] and their role in the induction of obesity [38] and diabetes [39-41]
indicates altered expression of multiple miRNAs in metabolic tissues [42]
Furthermore miRNAs such as miR-34a [43] and miR-122, miR-132 [44,45]
that directly inhibit Sirt 1 are associated with poor activation of hepatic
genes involved in glucose and lipid metabolism [46] with an increase in
acetylated p53 involved with cell apoptosis and NAFLD [47,48]. The p53
effects on miR-34a transactivation involve Sirt1 expression associated
with insulin resistance and the development of metabolic disease [49,50].
Nutrigenomics diets maintain Drug and Insulin Therapy
with the Prevention of Type 3 Diabetes
Interest in the nature of food intake has increased since the nature of
fat consumption (low or high) may require further evaluation and may
contain xenobiotics with marked effects on neuronal apoptosis and
neuroendocrine disease [17].The effects from environments in developing
countries (urban inhabitants) contain xenobiotics (soil, water, air) that
may contribute to Type 3 diabetes. High fibre diets [51] that contain
fruit and vegetables have become important for the treatment of NAFLD
with the reduction in the absorption of lipophilic xenobitics with the
prevention of Type 3 diabetes. Activation of hepatic nuclear receptors
such as the Sirt1/pregnane X receptor (PXR) by calorie restriction (low
glycemic index/fatty acid consumption) are important to the science of
nutrigenomics [17] with relevance to activation of hepatic xenobiotic
metabolism connected to the prevention of neuronal apoptosis.
The consumption of fruits such as apples that contain pyruvic acid (450
mg/apple) an antioxidant and an activator of Sirt1 [52-54] is essential for
diabetes treatment. Other brain nutrients to prevent Type 3 diabetes to
maintain brain glucose homeostasis include phosphatidyl inositol and
leucine both important for the maintenance of Sirt 1 related neuron
function. Exercise and heavy work activities may rapidly deplete brain
pyruvic acid, leucine and phosphatidyl inositol with the acceleration of
brain insulin resistance [55]. Excessive ingestion of vegetables not more
than (1-2 gm/day) may cause suprachiasmatic disorders with accumulation
of brain phytosterols with aging [51]. The nature of phytosterols that
regulate liver cholesterol metabolism are critical to prevent and reverse
NAFLD with HDL cholesterol metabolism closely linked to phytosterol
ingestion [51]. Specific polyphenols found in vegetables and fruits need
careful evaluation since high doses may cause increased oxidative stress
with toxicity to the liver and induction of NAFLD and chronic disease
[13]. Nutritional science that involves the maintenance of Sirt 1 regulation
of DNA repair require effective function of neuronal telomeres and
ingestion of therapeutic foods is essential to prevent Type 3 diabetes [29].
Nutrigenomic diets have become important to prevent Sirt 1 inhibition
with diets rich in palmitic acid and alcohol discouraged and both are
inhibitors of Sirt 1 [29]. Butyric acid is a histone deacetylase inhibitor
used in the treatment of diet induced insulin resistance [56,57] with doses
required to reduce amyloid beta protein aggregation in AD but butyric
acid may completely inhibit the Sirt 1 deacetylase activity essential for cell
telomere growth and mitochondria survival [24].
Sirt 1 is essential for maintenance of insulin therapy in diabetes and
aging [58-60]. In diabetes interest in the hepatic drug transport has
accelerated with toxic effects of drugs on insulin therapy. More than 600
drugs (eg: Asprin, Warfarin, Furosemide, Atrorvastatin, Clopidogrel,
Levothyroxin Acetaminophen, Cholecalciferol, Simvastatin) have been
listed that corrupt insulin therapy with drug-drug interactions involved in
diabetes [61]. Interests in nutrigenomic diets and drug transport indicate
the importance of Sirt 1 in brain to liver drug transport pathways via
the blood brain barrier [62-66] and require hepatic Sirt 1 regulation of
glucose, drug, cholesterol and bile acid metabolism [24]. Diabetes and
hepatic and brain amyloid beta metabolism [29] are connected to drug
metabolism (Figure 3) and defective hepatic drug metabolism may be
the primary defect in diabetes with acceleration of neuronal apoptosis by
toxic brain amyloid beta metabolism secondary to Type 3 complications
associated with Type 1 or Type 2 diabetic disease [29].
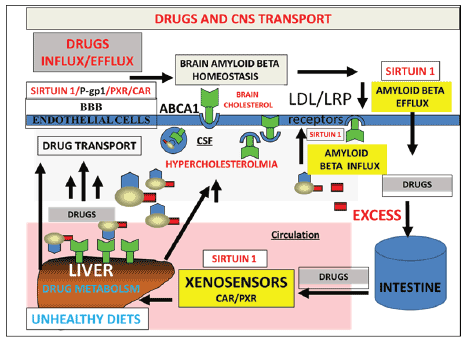
Figure 3: Unhealthy diets such as high fat diets downregulate hepatic drug metabolism with the corruption of insulin therapy in diabetes and aging.
Amyloid beta metabolism in the brain and liver are connected to drug metabolism and defective hepatic drug metabolism may be the primary defect
in Type 3 diabetes with acceleration of neuronal apoptosis by abnormal brain amyloid beta metabolism associated with Type 1 or Type 2 diabetes.
Abbreviations: P-gp1, P glycoprotein, PXR, pregnane X receptor, CAR, constitutive androstane receptor, ABCA1, ATP binding cassette transporter
1, BBB, blood brain barrier, LDLr, low density lipoprotein receptor, LRP, LDL receptor related protein.
In recent finding approximately 40 miRNAs have been shown to be
dysregulated [67-69] in diabetic individuals. The identification of specific
dietary supplements that may regulate DNA or microRNA metabolism
has become important with nutritional interventions essential for rapid
reversal for the severity of diabetes. Diets that do not contain therapeutic
food supplements promote Sirt 1 downregulation and disturb p53/mi
RNA metabolism [70] with relevance to toxic effects of drugs on insulin
therapy in diabetes. Several miRNA have been shown to effect drug
metabolism with nuclear receptors involved in the regulation of drug
and xenobiotic metabolism [71]. Elevated xenobiotic cell levels have been
shown to interfere with miRNA expression [72] with relevance to nuclear
Sirt 1 activity.
Nutrigenomic diets activate Sirt 1 and accelerate the hepatic metabolism
of toxins such as bacterial lipopolysaccharides (LPS) and mycotoxins that
inhibit Sirt 1 and promote insulin resistance with relevance to brain, liver
and pancreatic disease [73]. Nutrigenomic diets that do not contain LPS
have become important since LPS effects on membrane asymmetry [74,75]
involve membrane calcium levels/flux that determine severity of insulin
resistance, abnormal drug transport with amyloid beta dyshomeostasis
[13] connected to Type 3 diabetes.
Conclusion
Nutritional interventions have become important for the reversal of
Type 3 diabetes associated with accelerated aging and linked to Type 1
and Type 2 diabetes. Therapeutic foods that contain anti-aging dietary
components activate the anti-aging gene Sirt 1 with relevance to cell
glucose dyshomeostasis linked to heart, pancreas, liver and brain diseases.
Nutrigenomic diets that activate hepatic Sirt 1 improve drug metabolism
with relevance to insulin therapy in diabetes. Lifestyle changes such as
drug, environment, diet and stress are involved with the metabolism of
therapeutic nutrients that are depleted from the brain with extended
workload/exercise treatment periods associated with the downregulation
of brain nuclear receptors with relevance to Type 3 diabetes and the
severity of insulin resistance in various global communities.
Acknowledgements
This work was supported by grants from Edith Cowan University, the
McCusker Alzheimer’s Research Foundation and the National Health and
Medical Research Council.
Article Information
Article Type: Short Commentary
Citation: Martins IJ, Calderón AM (2016) Diet and Nutrition reverse
Type 3 Diabetes and Accelerated Aging linked to
Global chronic diseases. J Dia Res Ther 2(2): doi
http://dx.doi.org/10.16966/2380-5544.117
Copyright: © 2016 Martins IJ, et al. This is an open
access article distributed under the terms of
the Creative Commons Attribution License,
which permits unrestricted use, distribution, and
reproduction in any medium, provided the original
author and source are credited.
Publication history:
Received date: 15 Feb 2016
Accepted date: 25
Feb 2016
Published date: 03 Mar 2016




