$
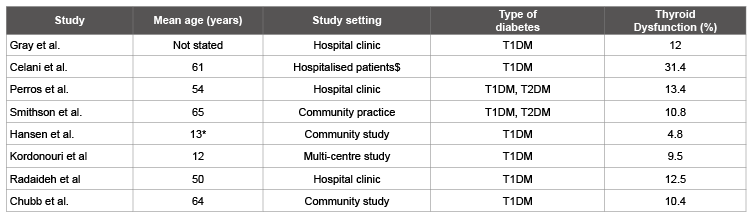
These were patients with poor glycaemic control; thyroid dysfunction mostly improved with correction of hyperglycaemia.
*Median. T1DM, type-1 diabetes; T2DM, type-2 diabetes

Sayantan Ray* Sujoy Ghosh
Department of Endocrinology and Metabolism, Institute of Post Graduate Medical Education & Research and SSKM Hospital, Kolkata, West Bengal, India*Corresponding author: Dr. Sayantan Ray, Department of Endocrinology and Metabolism, Institute of Post Graduate Medical Education & Research and SSKM Hospital, Kolkata 700020, West Bengal, India, Tel: 91-9231674135; E-mail: sayantan.ray30@gmail.com
Thyroid disease and diabetes are known to be pathophysiologically associated. These associations have clinically relevant implications for insulin sensitivity and adequate management requirements. Interconnections of common signalling pathways forms the underlying basis of this association. Unrecognised thyroid dysfunction may impair metabolic control in patients with diabetes. Interactions between thyroid hormone and the basal mechanisms controlling appetite, energy expenditure and insulin sensitivity regulation are also important areas to explore. The pathophysiological mechanisms underlying this linked regulation are increasingly being unravelled. A clearer understanding of this multifaceted relationship between diabetes mellitus and thyroid disease has the potential to assist in optimization of treatment in diabetic patients. However, there is no consensus regarding optimal thyroid screening strategies in routine diabetes care.
Thyroid dysfunction; Diabetes mellitus; Associations; Pathophysiology; Thyroid screening
Both thyroid diseases and diabetes mellitus (DM) are commonly encountered in clinical practice. They can influence each other and the associations between these two conditions have been reported by earlier studies [1]. An in-depth underlying relation between DM and thyroid dysfunction exists. A growing body of evidence pointed towards an array of complex interlinking biochemical, genetic, and hormonal malfunctions reflecting this pathophysiological association [2,3].
It has been found that thyroid dysfunction is more common in patients with DM than in the normal population in studies done in various settings [4-11] (Table 1). The prevalence of thyroid disorder in diabetic population was found to be 13.4% with the highest prevalence in females with type 1 DM (31.4%) and lowest prevalence in males with Type 2 DM (6.9%) [6]. A recent meta-analysis that was conducted on all available data in 10, 920 patients with DM reported a mean frequency of thyroid disease of 11% with no difference between T1DM and T2 DM [12]. Thyroid disorders were found to be more common in T1DM subjects as compared to those with T2DM. Autoimmunity is thought to be the major cause of thyroid-dysfunction associated DM [9,13]. The complex relationship between thyroid disease and DM has clinical implications. Treatment of thyroid dysfunction in diabetic patients will benefit glycemic control, reduce cardiovascular risk, and improve general well-being, nevertheless consensus regarding optimal thyroid screening strategies in routine diabetes care is still lacking.
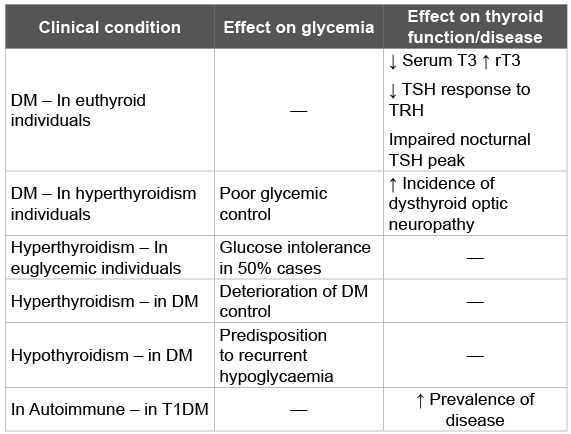
Hyperthyroidism is known to promote hyperglycemia [14]. Glucose intolerance of variable degree is observed in nearly 50% of patients with Graves’ disease and overt diabetes occurs in 2-3%, when normal individuals develop hyperthyroidism. It is well recognized that diabetic patients with hyperthyroidism experience deterioration of their glycemic control and thyrotoxicosis has been shown to precipitate diabetic ketoacidosis [15]. On the other hand, recurrent hypoglycemic episodes provide clues towards the development of hypothyroidism in patients with T1DM and replacement with thyroid hormones has shown to reduce the fluctuations in blood glucose levels [16]. It is well-recognized that both clinical as well as subclinical hypothyroidisms (SCH) can produce insulin resistant (IR) states. Impaired insulin stimulated glucose utilization in peripheral tissues was thought to be the underlying mechanism of IR indifferent In vivo and In vitro studies [17-19].
Additionally, an increased risk of nephropathy was found in T2DM patients with SCH which may be related to the decrease in cardiac output and increase in peripheral vascular resistance observed with hypothyroidism and the consequent decrease in renal flow and glomerular filtration rate [20, 21]. In 2005, Den Hollander et al. had shown that treating hypothyroidism improved renal function in patients with DM [22]. Regarding retinopathy, a recent study by Yang et al. reported that diabetic patients with SCH have more severe retinopathy as compared to euthyroid patients with diabetes [23]. Greater risk of nephropathy and retinopathy seen in diabetic patients with SCH provides justification of screening patients with T2DM for thyroid dysfunction and treating when detected.
Diabetes is a prime risk factor for cardiovascular disease (CVD). Hypothyroidism may increase this risk through its independent associations with atherosclerotic heart disease. In patients with hypothyroidism CVD risk may be enhanced through multiple interactions with CVD indices like dyslipidaemia and hypertension, features that overlap with the typical phenotype of IR [24]. The blood pressure changes, altered lipid metabolism, decreased cardiac contractility, and increased vascular resistance that occur in hypothyroidism are attributable to diminished thyroid hormone action on multiple organs such as the heart, liver, and peripheral vasculature and can be reversed with thyroid hormone (TH) replacement [25].
Table 1: Prevalence of thyroid dysfunction in patients with diabetes mellitus [4-11]

$
These were patients with poor glycaemic control; thyroid dysfunction mostly improved with correction of hyperglycaemia.
*Median. T1DM, type-1 diabetes; T2DM, type-2 diabetes
Studies done in hypothyroid patients showed elevated HbA1c not only in the presence of diabetes but also in non-diabetic subjects [26]. Altered erythrocyte life span due to low RBC turnover in hypothyroid patients may be partially responsible for spurious elevation in HbA1c levels [27]. Hence the role of HbA1c as a marker of diabetes was questioned in such conditions specially when American Diabetes Association (ADA) has endorsed it as diagnostic criteria for DM.
In euthyroid individuals with DM the serum tri-iodothyronine (T3) levels, basal thyroid - stimulating hormone (TSH) levels and TSH response tothyrotropin releasing hormone (TRH) are all subjected to alteration by the glycemic status. DM appears to influence thyroid function at two sites; firstly at the level of hypothalamic control of TSH release and secondly at peripheral tissue by converting T4 to T3. The nocturnal TSH peak is blunted or abolished in diabetic patients, and the TSH response to TRH is also impaired [28]. Reduced T3 levels have been observed in patients with uncontrolled diabetes. Possible explanation for this “low T3 state” could be impairment in peripheral conversion of T4 to T3 that normalizes with improvement in glycemic control. Higher levels of circulating insulin coupled with IR have shown a proliferative effect on thyroid tissue which may lead to larger thyroid size with increased nodule formations [29,30]. Efficacy of thyroid hormone treatment in hypothyroidism may be affected by co-existent diabetes. A higher prevalence of TIDM is observed in patients with Graves’ orbitopathy (GO) than in the normal population. Higher incidence of dysthyroid optic neuropathy (DON) has been documented in diabetic subjects with GO compared to nondiabetics [31].
Both DM and thyroid disorder may affect the health of mother and foetus with impact on obstetric care. High Anti-TPO antibody (TPO Ab) titres have been documented in pregnant women at risk for gestational DM [32]. Postpartum thyroid dysfunction occurs in upto 25% of women with T1DM [33]. Table 2 summarises how DM and thyroid diseases can mutually influence other disease process.
This biochemical diagnosis accounts for a large proportion of thyroid dysfunction encountered in diabetic patients. Perros et al. reported a prevalence rate of 5% in the hospital outpatient setting [6], whereas in community-based female diabetic patients a prevalence of 8.6% was reported by Chubb et al. [11]. The implications of SCH in the patient with diabetes will likely depend on its probability of progression to overt disease, its impact on metabolic control of diabetes, and the potential for therapeutic benefits with levo-thyroxine (LT4).
Epidemiological studies suggest a common genetic background for both thyroid disease and DM. Still, the identification of common genes is currently restricted almost entirely to autoimmune etiologies. Among autoimmune conditions in human, the strongest association is observed between T1DM and autoimmune thyroid disease (AITD) [34]. Familial clustering is also seen frequently. The prevalence rate of autoimmune thyroiditis in relative of T1DM may reach 48% compared to 3–10% in the general population. Both AITD and T1DM showed individual association with various HLA class II sequences. Even with this strong genetic association, information on shared susceptibility genes for T1DM and AITD is deficient Apart from the MHC locus, a good number of other genes have recently been suggested to be related with an elevated risk for both conditions [35].
Although there is a similar frequency of thyroid disease associated with T2DM, genetic links are not well characterized. Few studies suggest a direct genetic basis. Current data on polymorphism of the de-iodinase type 2 (DIO2) gene, Thr92Ala, demonstrate that homozygosity for this polymorphism is connected with an increased risk of T2DM [36]. Supported by a meta-analysis in almost 11, 000 individuals, these data point out a possible role of intracellular T3 on insulin sensitivity [12].
Table 2: Diabetes mellitus and thyroid diseases: mutual influence on other disease process
THs have well-recognized effects on glucose and lipid metabolism, [37] both by short- and long-term interaction with the regulatory pathways for energy homeostasis and through direct interaction with insulin regulation and glucose disposal in peripheral tissues. Recently, a role for THs and TRH in the central thermoregulatory pathways has been identified. In addition to TH nuclear receptors, TRH neurons in the hypothalamus express melanocortin receptor type 4 (MC4R) also. This receptor is involved in central energy regulation [38]. Activation of MC4R leads to reduction in food intake and increase in energy expenditure [39].
AMP-activated protein kinase (AMPK) is a cellular energy sensor that mediates the effects of various hormonal and nutritional signals in the hypothalamus. Recently, the hypothalamic control of peripheral metabolism through AMPK has been recognized. Inhibition of hypothalamic AMPK reduces glucose production in the periphery [40]. Moreover, AMPK links glucose regulation to fatty acid (FA) synthesis via the carboxylation of acetyl-CoA, which is then catalysed by acetylCoA carboxylase. Recently, Lopez et al. demonstrated reduction the activity of hypothalamic AMPK in hyperthyroidism or with central administration of T3 [41]. THs could alter glucose metabolism indirectly by way of their interaction with various hypothalamic signals. However, the exact mechanisms underlying this complex interaction remain to be elucidated.
THs can influence carbohydrate mechanisms through its interaction with adipocytokines and gut hormones. Circulating ghrelin levels show inverse relation with T3, independent of the forms of stimulation [42]. Ghrelin modulates insulin sensitivity and has effects on islet cell proliferation. Hyperthyroidism is associated with IR and hyperinsulinemia suppresses ghrelin levels [43]. Heterogenous effects of THs have been reported on adipokines, particularly leptin. Tumor necrosis factors (TNF) elevated in hypothyroidism, serves as one of the major adipokines which contributes to IR, a decrease in glucose disposal and the striking increase in FAs [44]. The interaction between THs and adiponectin remains to be clarified and available studies have shown inconsistent results.
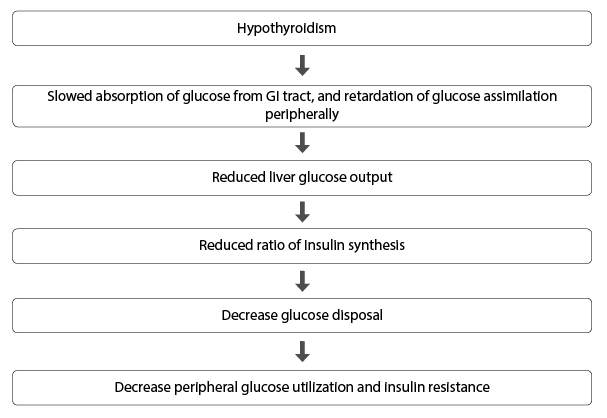
Figure 1: The relation between hypothyroidism and insulin resistance
TH actions on peripheral tissue further describe the overall effects of thyroid function on insulin secretion, action and glucose uptake. In hypothyroid individuals, there is a decline in glucose-induced insulin secretion by β-cells, and the β-cells response to glucose or catecholamine is increased in hyperthyroidism possibly due to increased β-cell mass. Moreover, insulin clearance is increased in patients with thyrotoxicosis [44,45].
Glucose intolerance in thyrotoxicosis is due to elevated hepatic glucose output together with upregulated glycogenolysis [3]. Therole of hypothalamic sympathetic action in liver has been suggested along with increased expressionof GLUT 2 transporters in liver which eventually cause elevation in plasma free FA [46]. This phenomenon is responsible for exaggeration of hyperglycaemia in T2DM.Thyrotoxicosis may also lead to ketoacidosis as a result of elevated lipolytic actions and increased hepatic β oxidation [47,48].
In diabetic patients, hypothyroidism may influence metabolic control via effects on glucose metabolism in contrast to those seen in hyperthyroidism. These effects include reduced hepatic glucose output, gluconeogenesis and peripheral glucose uptake. This ultimately leads to a predisposition to hypoglycaemia [49]. Frequent hypoglycemia was documented in children and adolescents with diabetes and SCH. In SCH, decreased rate of insulin stimulated glucose transport caused by perturbed expression and translocation of GLUT 2, may lead to IR [50]. Besides, hypothyroidism is associated with endothelial dysfunction as determined by impairment in flow-mediated vasodilatation [51]. A study in healthy euthyroid men found positive correlations between TSH, endothelial dysfunction and IR [52] providing further evidence to the three-way relationship between thyroid status, insulin resistance and CVD risk. The relation between hypothyroidism and insulin resistance is shown in Figure 1.
THs stimulate catecholamine action which leads to increase in adipose tissue lipolysis, thereby increasing circulating FAs [53]. Elevated circulating FA concentrations together with an increased availability of gluconeogenic substrates from peripheral sources may also clarify the marked increase in gluconeogenesis in T3-treatedanimals.The various genes which influence the interaction of TH and skeletal muscles consist of GLUT1, GLUT4, β2 adrenergic receptors, PPARγ coactivator-1α (PGC- 1 α), phosphoglycerate kinase (PGK), and mitochondrial uncoupling protein. Among the different genes identified, GLUT-4 and mitochondrial uncoupling protein 3 (UCP-3) have been studied in detail [46,54-56]. In the skeletal muscles, T3 can elevate basal and insulin mediated transport of glucose by modulating GLUT 4. A recently identified gene UCP 3 has been proposed to be linked with glucose metabolism and decreased FA oxidation. It may have a critical role in the AMPK signalling also [17,57].
The diagnosis of hypothyroidism in diabetic patients based only on clinical manifestations is not easy. The association of low TH levels with acute hyperglycaemic states may pose difficulty in the accurate interpretation of thyroid function tests (TFTs) in patients with uncontrolled diabetes. Typical alterations include a low serum T3 due to impaired extra thyroidal T4 toT3 conversion, a low serum T4 caused by decreased protein binding, and a low serum TSH level. Severe diabetic nephropathy can wrongly be attributable to hypothyroidism as individuals with this condition may also have pallor, oedema, fatigue, and weight gain.
Highly sensitive immunoassay for serum TSH (detection limit of <0.1 mU/l) is the most reliable and sensitive screening test for hypothyroidism. Furthermore, subclinical thyroid dysfunction can only be diagnosed by an abnormal TSH because the serum T3 and T4 are normal and the patients are generally asymptomatic [58].
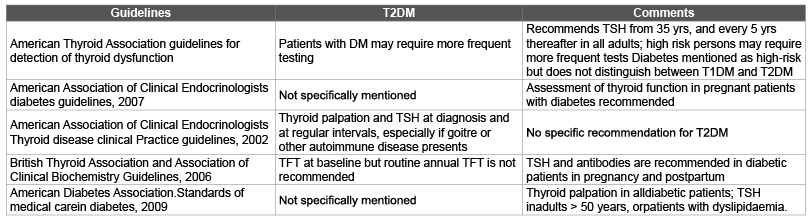
The close interactions between thyroid status and metabolic control favours close monitoring of thyroid function particularly patients T1DM patients. However, the case for annual screening in patients withT2DM is less precise. The available guidelines are either not specific regarding routine monitoring [59,60] or unequivocally recommend against routine annual screening in T2DM [61]. The American Thyroid Association (ATA) guidelines recommend frequent testing for thyroid dysfunction for T2DM patients. High-risk patients may require more frequent testing [62]. The main discrepancies relate to the choice of TFTs, the intervals between testing, whether routine screening is indicated in all diabetic patients, and whether a specific screening policy is necessary. These uncertain ties are reflected in the guidelines published by the major endocrine and diabetes societies on thyroid disease screening (Table 3).
Table 3: Recommendations from major diabetes and endocrine practice guidelines on thyroid screening in patients with Type 2 diabetes
In a recent review by Kadiyala et al. [12] simplified approaches have been proposed. Authors suggested measurement of TPO Ab and TSH in all patients with diabetes at baseline, and subsequent annual testing for only those patients with T1DM, having positive antibodies, or TSH concentration in the upper limit of normal. The simplified algorithm is elaborated in Figure 2.
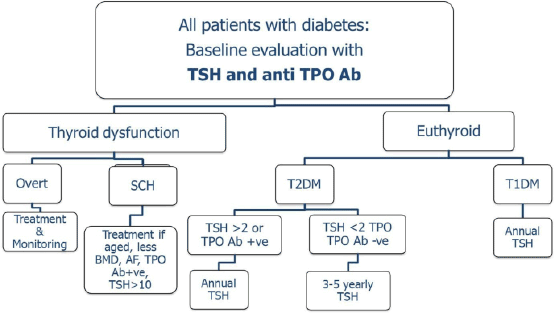
Figure 2: A simplified screening and monitoring algorithm for thyroid dysfunction patients with diabetes (modified from Ref. 12). SCH, subclinical hypothyroidism; BMD, bone mineral density; AF, atrial fibrillation; TPO, thyroid peroxidise.
Metformin is considered as a first-line drug for treatment of T2DM. In a small retrospective study, metformin suppressed TSH to subnormal levels, without changes in Free T4 and T3 levels [63]. Prospective studies in patients with diabetes and hypothyroidism on stable LT4 treatment showed that during metformin administration for 3 months, TSH levels were significantly lower than basal TSH concentrations with reverse effects occurred on discontinuation of metformin [64,65]. A recent study in patients with benign thyroid nodules has demonstrated significant decrease in nodule size with metformin in patients with IR [66]. The effect of metformin, which was produced over a 6-month period, associated with a fall in TSH concentrations and achieved a reduction amounting to 30% of the initial nodule size when metformin was administered alone and up to 55% when it was added to LT4 treatment.
From the above studies, it appears that metformin has suppressive effect on TSH secretion in hypothyroid patients, an effect that seems to be independent of LT4 treatment and does not alter the TH profile. A rebound of TSH secretion usually occurs at about 3 months after metformin withdrawal.
THs have profound influence on diverse physiological processes including metabolism of lipid, protein, and carbohydrate. TH analogues have made possible the development of novel strategies in the management of atherosclerosis, diabetes and obesity [67]. Search for the potent thyroid hormone analogues that electively elude the harmful effects of TH, and simultaneously produce desirable therapeutic effects is currently the centre of attention. [3,68]. Recent investigations and subsequent findings have provided many cues that could unravel trails of complex physiological mechanisms in the endocrine crosstalk of hyperglycaemia and thyroid dysfunction.
Thyroid dysfunction is common in patients with DM. The association between thyroid disorders and DM is characterized by complex interdependent interactions. The underlying pathophysiological mechanisms are increasingly being unravelled. Untreated thyroid dysfunction may impair metabolic control inpatients with diabetes and also may magnify the existing CVD risk. It is also evident from the existing literature that IR plays a crucial role in connecting T2DM and thyroid dysfunction. Novel molecules have paved the path for the development of suitable thyroid hormone analogues to treat associated metabolic diseases. The increased occurrence of thyroid dysfunction in diabetes need for a systematic approach to thyroid testing. Until now, screening practices vary widely and specific guidelines are lacking. The relationship between T2DM and thyroid disorders has been a less explored area which may behold answers to various facts of metabolic syndrome and related cardiovascular disorders.
Download Provisional PDF Here
Article Type: Research Article
Citation: Ray S, Ghosh S (2016) Thyroid Disorders and Diabetes Mellitus: Double Trouble. J Dia Res Ther 2(1): doi http://dx.doi.org/10.16966/2380-5544.113
Copyright: © 2016 Ray S, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access