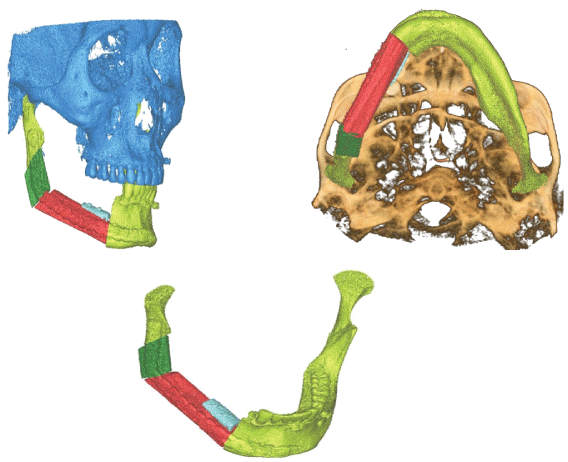
Figure 1: Mandible reconstruction.


Saddam Noman AL-wesabi1,2 Eissa Abdo Al-shujaa1,2 Yueyan Zheng1 Xie Fu Qiang3*
1Lanzhou University School of Stomatology, Oral and Maxillofacial Surgery Department, Gansu Province, China*Corresponding author: Xie Fu Qiang, Department of Oral and Maxillofacial Surgery, Lanzhou University Second Hospital, Lanzhou University, Lanzhou, Gansu Province, China; E-mail: xiefq@lzu.edu.cn
The mandible supports the oral cavity's soft tissues and teeth. The treatment of mandibular segmental defects must consider the missing component’s anatomic; the gold standard of mandibular reconstruction replaces the defect with vascularized bone unless the perioperative risk is extremely great owing to comorbid conditions. To achieve bony healing and restore the mandibular contour, rigid fixation of the free flap bone segments to the remaining mandible is important. The reconstruction made it easier to meet a wide range of treatment requirements. The most common reasons for reconstructive surgery today are defects after tumor surgery due to benign or malignant neoplasms of the oral cavity. First-line or adjuvant radiation plays a major role in today's multidisciplinary head and neck cancer treatment. When exposed to radiation, the mandible, in particular, is susceptible to osteoradionecrosis. So, this article review is to review the history of oromandibular reconstruction, the biomechanics of the mandible and summarizes the various reconstruction options available for the mandible, including defect classification, goals in reconstruction, donor sites, current reconstructive options, dental rehabilitation, and persistent associated problems.
Mandibular reconstruction; Cone-beam computed tomography (CBCT); Reconstructed mandible complications; Fibula fable
Extirpative surgery for malignant or benign diseases is the most common reason for oral and maxillofacial reconstruction. Oral and maxillofacial reconstruction may be necessary as a result of other diseases. These include anomalies caused by severe trauma, congenital deformities, tissue destruction caused by infectious or autoimmune diseases, and therapy-related complications (e.g., osteoradionecrosis). Defect classification is needed for optimal communication, treatment planning, evaluation of functional and esthetic outcomes, and prognosis for functional recovery. Many things go into reconstructive surgery that isn't covered by traditional oncologic classification systems, like how long a person can live [1- 3]. There are a few essential principles to observe when considering the reconstruction of oral and maxillofacial defects. In each case, the biology of the tumor and the geometry of the defect should be taken into account. The patient's aspirations and goals must align with the functional restoration objectives. For example, it may not make sense to plan a reconstruction that includes dental rehabilitation for a patient who has been edentulous for a long time and has never sought treatment. The risks-benefits analysis must take into account comorbid conditions. Free tissue rebuilding may extend the procedure, increasing the risk of general anesthesia and difficulties afterward. A less taxing reconstructive approach would benefit several patients with significant comorbidities. Bodily habits, prior surgery history, peripheral vascular disease, and patient occupation can all limit reconstructive options by limiting the use of specific donor sites. All of these factors make it difficult to use a rigorous reconstructive procedure in every scenario. Rather, due to the wide range of options in the reconstructive “toolbox” and the unique and creative approach to each condition, head and neck reconstructive surgery is more of an art than a science.
Each case has its unique plethora of challenges to solve and goals to achieve in terms of reconstruction. The options available are restoring function, cervicofacial symmetry and form, and restoration of cervicofacial symmetry and shape. Between cavities and regions of the head and neck that should not interact, barriers are produced. The face is resurrected. With dental rehabilitation and re-establishment of sensation in a "perfect reconstruction," the lost tissue is replaced with an exact substitute. This is a difficult, if not impossible, task to achieve. During the last decade, researchers could transfer parts of the head from one person to another via vascularized composite allotransplantation. The first of these transplants were conducted in 2005, and about 25 partial or full-face transplants have taken place since then. Although advances in microsurgical techniques, organ transplantation, and immunosuppression have made great progress in this field, the many hurdles and issues associated with this approach prevent its wide use. Tissue structure and function should be evaluated whenever possible, and like with like, it should be replaced. An innervated muscle or a static extension of a functional muscle can be used to restore movement. Tissues with similar properties and appearance replace the mucosa and skin. Bones aid in the structural stability of the midface and mouth cavity. The mandible should be replaced with vascularized bone, or the remaining bone segments should be rigidly fixed to resist mastication forces. The maxillary bone can take less pressure, and, in some cases, soft tissue can be used to replace it without a noticeable change in appearance. The defect's functional qualities, on the other hand, should be taken into account when reconstructing the flaw. Solid dental repair and orbital content support may require the bone. The correct categorization of the maxillary deficiency is critical for determining the best reconstructive choices (Figure 1).

Figure 1: Mandible reconstruction.
The mandible is a U-shaped bone with rear vertical extensions that determine the lower face's height, width, and projection. The arch and body of the mandible are the primary teeth-bearing areas. Simultaneously, the ascending ramus with condyles articulates posteriorly with the skull base, facilitating important tasks such as speaking and chewing.
The mandible’s bone abuts the chin, lips, and oral commissures in the front; the mandible’s bone joins the external cheek and intraoral lining in the middle; the mandible’s bone touches the pharyngeal pillars in the back, and the mandible’s bone medially abuts the mouth floor and the tongue. Resection of tumors in this area may result in bone defects and the loss of soft-tissue components in the surrounding area. As a result, a mandibular defect classification system must also include soft-tissue anomalies.
The mandibulectomy defect classification system consists of a Roman numeral, a subdividing letter, and, in subcases, a subcategory number. The Roman numerals indicate the bone defect (I, II, and III). The anterior arch is affected by type I abnormalities, which always include the symphysis but may also include one or both parasymphyses (Figure 2).
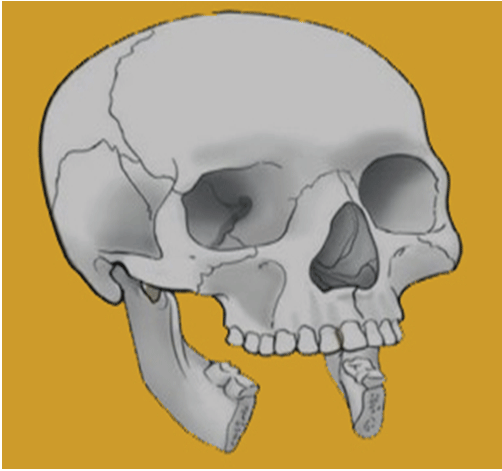
Figure 2: Type I anterior I bony defect.
Type II hemimandible defects always include a portion of the body, the angle, and the ascending ramus (with or without the condyle) (Figure 3).
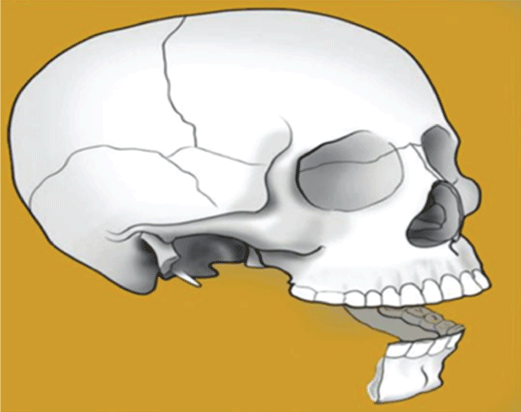
Figure 3: Type II bony defect, hemimandible.
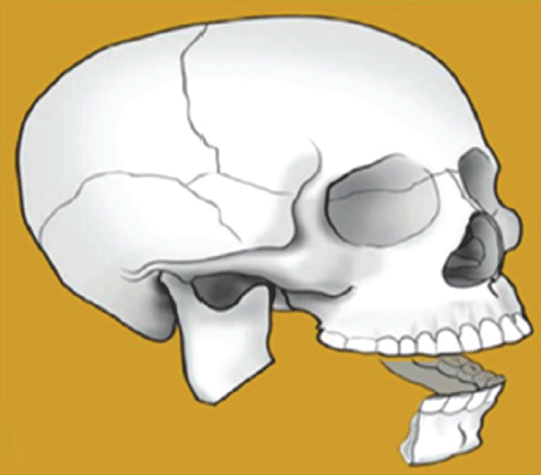
Figure 4: Type III bony defect, lateral.
Type III flaws are lateral defects that damage one or all three parts of the ascending ramus, angle, or body (Figure 4). A letter indicates the severity of the soft-tissue defect and the involvement of any combination of skin, subcutaneous tissue, muscle, intraoral structures, and mucosal lining (A, B, C, D).
• A indicates that there is no soft-tissue defect.
• B indicates a defect in intraoral structure and mucosal lining.
• C indicates simply a skin/external soft-tissue defect.
• A through-and-through or intraoral structures/lining and skin defect is denoted by the letter D.
The extent of the intraoral defect has been found to govern the reconstruction algorithm for type II hemimandibular defects; thus, a sub-classification of B1 and B2 is presented. The buccal mucosa, the
floor of the mouth, the palate, the tongue, and the pharynx are five intraoral anatomical zones.
• B1 refers to intraoral zone excision including two or fewer zones.
• B2 refers to the excision of three or more intraoral zones.
As a result, a 13-zone classification system is described (Figure 5).
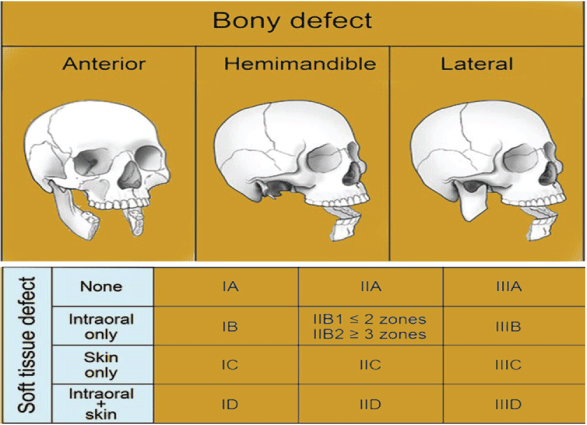
Figure 5: Mandibulectomy defect classification system.
• 1A, 1B, 1C, 1D
• IIA, IIB1, IIB2, IIC, IID
• IIIA, IIIB, IIIC, IIID
The location of the resection bone must be considered first, followed by the required soft-tissue deficits for reconstructing each type of mandibular defect [4].
Dental rehabilitation is only possible in patients who have had their bones restored to a certain extent:
The bone implanted might be a xenograft (bone from another species), an allograft (bone from another person), or an autograft (bone from the patient) and be free (bone from another part of the same individual receiving the graft). Vascularized (free tissue transfer) or nonvascularized bone grafts are available (free bone grafts) [5,6].
There are three types of bone grafts: allogeneic, xenogenic, and autologous. Due to the risk of disease transmission and rejection responses, allogeneic and xenogenic transplants are less critical. Autologous bone transplants have been used to restore mandibular deformities in a variety of ways. For severe lesions, however, free bone transplants have downsides in terms of resorption, engraftment, and infection [7]. The gold standard for restoring bone level before dental rehabilitation is free bone grafts using osseointegrated implants for minor alveolar defects. Because of the limited blood supply through diffusion, there are only a few reasons for restoring mandibular continuity with nonvascularized grafts. Grafts may be obtained from various sites [8]. The cancellous and cortical bone structures in the respective grafts are different-the incidence of osteoconductivity increases when there is a high percentage of cancellous bone. The presence of supporting plates or meshes in cancellous bone indicates the presence of supporting plates or meshes [9-11].
Because cortical bone has a high osteogenic ability, it has limitations in engraftment. Bone grafts may theoretically be extracted from practically any human bone. Intraoral and extraoral donor locations might be subdivided. Bone is often removed from the symphysis, ramus body, or zygomatic bone through intraoral [12-17]. The cranium, ilium, tibia, and femur are an example of extraoral donor sites (Table 1) [18-22]. Due to the high, no graft achieves the optimal blend of stability and engraftment for mandibular repair. As a result, severe indications should be considered, particularly in jaws that have been irradiated or are otherwise compromised [23,24]. Intraoral Procedures An extraoral technique was used to transplant the symphysis. Ramus graft of the body Bone transplant on the cheek Calvarial bone transplant with split-thickness and complete thickness. The bone graft of the anterior/posterior iliac crest a costochondral graft is a graft that replaces the costochondral bone transplant in the femur with a graft that replaces the costochondral bone transplant in the tibia.
| Table 1: Intraoral and extraoral donor sites for nonvascularized bone harvesting |
|
| Intraoral Approach | Extraoral Approach |
| Symphysis graft | Calvarial bone graft (split-thickness/full thickness) |
| Body ramus graft | iliac crest bone graft (anterior/posterior) |
| Cheekbone graft | Costochondral graft |
| Femur bone graft | |
| Tibial bone graft | |
Table 1: Nonvascularized bone harvesting intraoral and extraoral donor sites.
The gap between revascularized free bone grafts was bridged using pedicled flaps. In the 1960s, they revascularized free bone grafts to repair mandibular continuity and awareness of the anatomic region's challenges after taking steps toward bone grafting. Because of their poor functional and cosmetic outcomes, as well as significant donor site morbidity, pedicled osteomyocutaneous flaps have been increasingly replaced by microvascular-free flaps. Because there are no other options for treatment, the indications are confined to salvage surgery. Pioneering work in the early 1970s using revascularized rib for mandible repair laid the foundation for today's microsurgical techniques [25, 26]. According to a study, single bone defects or composite tissue defects of the lower face can be repaired in a reliable and generally safe method [27]. In an irradiated condition, engraftment using vascularized free bone grafts was also possible. The iliac crest, scapula, fibula, and other bones were the most common and prospective donor sites. Today, they are regarded as the gold standard in mandibular reconstruction. With a success rate of over 90%, vascularized osseous-free flap repair has proved to be a safe and effective method [3,28-30]. Titanium reconstruction plates are used to safeguard the free vascularized bone graft at first, which is a common feature of all procedures [31].
Type of revascularized bone: A. Iliac crest free flap: Many factors can induce oromandibular deformities, including tumor removal, trauma, osteoradionecrosis, and a kind of osteomyelitis. These abnormalities might affect soft tissues only, or they can damage bone structures too though [2,32]. Using a multistage transfer of clavicle tissue enclosed in a tubed skin flap, the vascularized composite flap was introduced in the early 1970s to enable more efficient and effective repairs of oromandibular deformities [33] or a rib pedicled onto the mammary veins from inside [34]. For the vascularized composite free graft, Daniel [35] was studied at multiple donor locations. Taylor and colleagues reconstructed the mandible with groinfree bone flaps in 1978 [34]. In 1979, he established the anatomical basis for osteomusculocutaneous flaps of the deep circumflex iliac artery (DCIA). The anatomical basis for free flaps was described by Taylor et al., including the DCIA’s primary channel and ascending and transverse branches. The position of the skin paddle and DCIA perfusion conditions for the iliac crest bony structures were reported. The numerous benefits of these flaps correspond to the need for optimum reconstruction. For starters, there is plenty of soft tissue and bone to extract. Because of their enormous vascular diameter, the flaps have a high graft survival rate, resulting in excellent vascular anastomosis and low donor-site morbidity.
Furthermore, contouring this flap to the shape of the mandible, including the mandibular angle, is rather simple, and there is sufficient bone to harvest for maxillary structural contouring. Despite this, only a small amount of tissue has been obtained so far; scapular and fibular flaps have comprised the backbone in oromandibular reconstructions [34]. The DCIA flap's low utilization rate is probably due to its more complex anatomical approach than other flaps. DCIA flap dissection takes a long time and requires a higher level of anatomical knowledge on the side of the clinician. At the anterior superior iliac spine (ASIS) level, there are several anatomical differences in the DCIA, resulting in more bothersome bleeding from the various branches that breach the muscle layer [34]. As a result, clinicians must thoroughly understand this perplexing anatomical system. We characterize the anatomical diversity of the DCIA flap using Korean adult cadavers. In 1989, Taylor was the first to describe the iliac crest free flap based on the Deep Circumflex Iliac Artery (DCIA) and Deep Circumflex Iliac Vein. (DCIV). According to Ramasastri et al., the ascending branch of the DCIA provides vascularization to the oblique muscle and skin, resulting in the composite iliac crest free flap used today [35].
The flap has distinctive features that have made it a popular therapeutic option, particularly for head and neck reconstruction. The fibula-free flap, the second most commonly used flap, is used when height is required. However, various harvesting difficulties, short and small-caliber pedicle vasculature, and the danger of donor-site morbidity have all contributed to a reduction in flap usage. The flap is now commonly referred to as a “third-level flap”, and it is only used by a small percentage of reconstructive surgeons on a regular basis. The flap, on the other hand, is perfect for treating head-and-neck issues like mandibular and maxillary reconstruction. The flap can be harvested with or without a skin paddle. When bone repair is required, especially in the mandible, the DCIA creates a huge piece of cancellous bone up to 15 centimeters long and 6 centimeters wide, which is normally replaced by the fibula. The fibula, on the other hand, is only responsible for supplying cortical bone. The DCIA is an excellent candidate for a big block of bone to reconstruct the fibula when the peroneal artery input is insufficient or the lower extremities have been injured.
The skin paddle, on the other hand, will thicken the flap, making the inset difficult. A second flap, such as the groin flap, which may be harvested in the same field as the DCIA flap and often has the same vascular trunk, is preferable. The groin is removed from the DCIA flap, resulting in a skin paddle that is smaller and easier to insert. The downside is that the DCIA and groin pedicles are frequently detached, resulting in the need for two extra microvascular anastomoses in one operation [36].
B. Fibula flap: Since the nineteenth century, the mandibular surgical reconstruction method had grown when various local flaps, regional flaps, and prostheses were utilized [37-41].
During World War II, free bone grafts, vascularized ribs, alloplastics, and prostheses were used to reconstruct the mandible [39]. Microvascular surgery (surgery on tiny blood vessels) influenced mandibular reconstruction techniques in the 1970s. The first donor preferred location was the vascularized rib. Still, it was problematic because of its insufficient bone stock and insufficient support for mastication forces and to support a prosthetic dentition [39]. Taylor and colleagues reported the vascularized fibula flap for the first time in 1975 [42]. The metatarsus was reported as a possible donor source in 1980, but the bone was found to break down due to direct trauma [39]. The use of free vascularized osseous flaps to reconstruct bony defects has become a reliable and preferred mandibular reconstructive surgical procedure [31]. Surgeons commonly harvested the fibula, scapula, or iliac crest [43]. A suitable flap should be chosen depending on the state of the mandibular bone and the surrounding soft-tissue defect [44]. In 1989, Hidalgo found success in fibula-free mandibular reconstruction, and the fibula-free flap has since become the standard treatment for vascularized bone [40]. The fibula is said to withstand multiple osteotomies, be easily sculpted, be of ideal size, length, and contour, and have a thin, pliable, and sizeable skin paddle for repairing intraoral and extraoral soft-tissue defects [37]. Also, it allows for postoperative rehabilitation using osseointegrated dental implants and prosthetic reconstruction [45]. Microvascular-free bone flaps enhance the surgical complexity and the amount of data required to prepare for the surgery before. While significant progress has been made in fibula-free flap reconstruction of the mandible to improve the surgical process, it is essential that preoperative examination, planning, and preparation be done for a successful surgical outcome, given the added complexity of the anatomy and installation of osseointegrated dental implants. Because imaging and advanced digital technologies (ADTs) are now available, there is an opportunity to improve preoperative planning. Mandibular fibular reconstruction is a difficult surgical treatment. In the past, a substantial discontinuity defect faced the surgeon with a severe, if not insurmountable, reconstructive challenge. With the introduction of microvascular reconstruction, mandibular reconstruction became more predictable. The fibula-free flap became the workhorse for mandibular discontinuity defect reconstruction. As a result of the projected mandibular reconstruction technique, new alternatives for oral rehabilitation developed with osseointegrated implants, but with the demand for higher precision and accuracy of the reconstruction. The need for surgical design and simulation came as a result of this challenge. The mandible is a functional and aesthetically important structure of the head and neck that helps with facial contour, speech production, mastication, and deglutition [46]. Surgeons focused on the procedure of preventing unsatisfactory postoperative surgical outcomes that might affect the patient's quality of life, such as facial deformities and loss of essential activities such as mastication and deglutition, in fibula-free flap mandibular reconstruction. Atrophy of the mandible, tumor resection, traumatic injury, or congenital deformities may need mandible reconstruction. The aim of mandibular reconstruction is to return the patient’s shape, function, and quality of life. The surgeon wants the patient to be full and functioning after surgery. The goal is to restore the bone's contour and reduce cosmetic deformity while restoring functional mastication, deglutition, articulation, and oral competence [47]. The surgeon must evaluate adequate imaging to understand the defects and pre-operatively design the operation with the support of a surgical team in mandibular reconstructive surgery [43]. To ensure sufficient blood supply to the flap and its associated skin, preoperative angiography and Doppler evaluation of the lower leg were performed in all cases. Regardless of the fact that mandibular resection is intended to save lives and eliminate life-threatening illnesses; patients’ quality of life is often affected [37]. Patients who haven't had good planning and communication frequently develop facial deformities and malocclusions, are left without a dentition, and lose essential activities like speech, chewing, and swallowing. Patients with poor self-perception and low self-confidence are commonly psychologically sensitive to depression as a result of these conditions [48].
Segmental defects of the mandible may be caused by malignant and benign tumors, infectious diseases, or trauma. Mastication, swallowing, and speech problems may be caused by the mandible’s continuity, the disruption of muscle attachments, and sensory and motor nerve innervation loss. If a mandible is not repaired, it may lead to significant functional, cosmetic, and social problems and a poor health-related quality of life [49,50]. Immediate reconstruction is preferred from a practical and aesthetic standpoint [51]. A reconstruction plate may be used to bridge the gap in the mandible. This is possible if enough soft tissue remains for a watertight primary closure. Reconstruction plates are easy to use and apply, but they have the disadvantage of becoming susceptible to complications such as screw loosening, plate fracture, plate exposure, and infection [52]. A considerable level of donor site morbidity is associated with the harvest of composite flaps [53] (Medical problems and complications at the recipient site are also common).
The most severe complications need a second surgical procedure under general anesthesia. The majority of these significant complications arise at the recipient site. These complications might arise early after the operation, within days or weeks, or much later, months or even years after it's over. Except in severe instances of flap loss, we've seen that early problems are usually resolved swiftly and seldom necessitate fixing plating. The fixing plate is almost often removed during late interventions. Late significant recipient site problems generally follow prolonged attempts at conservative, noninvasive therapy to avoid surgery. In addition to the final surgical intervention, these protracted treatments are a significant burden on the patient and the hospital's resources. Some research tried to identify variables that increase the risk of postoperative complications. They describe mandible flaps reconstructions using additional donor locations such as the radial forearm or iliac crest and different recipient sites in the head and neck [54,55]. (A few publications focused on mandible reconstruction using fibula-free flaps.) On the other hand, these articles do not distinguish between complications at the recipient and donor sites, moderate and severe complications, or early and late complications [56].
According to Bähr W [57], the fibula fragments had a minimum size of 2.0 cm. (Titanium fixation plates were used to fix the fibula fragments.) The phrase “major recipient site complications” described issues that needed general anesthesia and surgical intervention. The time between the initial observation of the complication in the outpatient department and surgical intervention and the duration between reconstruction and surgical intervention were both recorded. Initially, the patient suffered from vascular complications such as bleeding, venous congestion, and insufficient perfusion of the skin island. Within days after the reconstruction, bleeding and venous congestion occurred, requiring immediate intervention. Skin necrosis and surgery to remove the dead skin were first used to treat insufficient perfusion of the skin island. When compared to nonsmokers, the estimated relative risk of smoking. Nicotine, and other carcinogens such as carbon monoxide and hydrogen cyanide, are found in cigarette smoke. Relative tissue hypoxia, aberrant cellular activity, and thrombogenesis are all caused by these byproducts. Nicotine causes vasoconstriction, which reduces oxygen accessible in tissues directly and indirectly. Complications in head and neck reconstructions are also linked to smoking [58]. (Continual smoking after mandibular reconstruction reduces tissue perfusion, which is compounded by decreased vascularization in the event of postoperative radiation).
The fixation plate, which was removed or sometimes refixed during the intervention, was virtually always the source of late recipient site problems. Major recipient site complications are common in mandibular reconstruction with free fibula flaps. Most early complications, which need intervention within six weeks, are vascular in origin [59]. We found that they are related to defects that cross the mandible's midline and need relatively complex reconstructions. Infection, wound dehiscence, osteonecrosis, and plate fracture are the most common reasons for later surgical interventions. This treatment is generally followed by months or even years of conservative treatment for the complications associated with continuing to smoke. Smoking cessation reduces the risk of late recipient site complications and should start as fast as possible after diagnosis and before any surgical oncologic treatment [60]. There has not been looked at the different effects of risk factors on early and late complications in mandibular reconstruction using free fibula flaps. Major recipient site complications appeared to be affected by the type of fixation plate used. However, we recommend against mini plates in the angular area because they may fracture during regular jaw activity when put there [61,62].
Our results reveal that the vascularized fibular free flap is a reliable method to reconstruct mandibular defects with an acceptable low morbidity rate, the challenge for the head and neck reconstructive surgeon, Oromandibular reconstruction is now very reliable and very successful with very real long functional and aesthetic outcomes using autogenous bone grafts and other current reconstructive options. Autogenous vascularized bone grafts combined with microsurgical techniques have revolutionized mandibular reconstruction in oral cancer surgery. Current mandibular reconstruction trends aim to restore a viable mandible with the correct form and a suitable maxillary, mandibular connection while reducing the requirement for invasive autogenous graft procurement. However, when it comes to reconstructive alternatives such as donor site selection, surgical time, and method of reconstruction, the ideal repair of mandibular abnormalities is still debatable.
This study is supported by the Cuiying Science and Technology Innovation of the Lanzhou University Second Hospital (CY2021- BJ-A18).
Download Provisional PDF Here
Article Type: REVIEW ARTICLE
Citation: AL-wesabi Saddam N, Al-shujaa Abdo E, Zheng Y, Qiang Xie F (2022) Literature Review of Mandibular Free Fibula Graft Reconstruction from Basic Concept to Complication. Int J Dent Oral Health 8(2): dx.doi.org/10.16966/2378-7090.392
Copyright: © 2022 AL-wesabi Saddam N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access