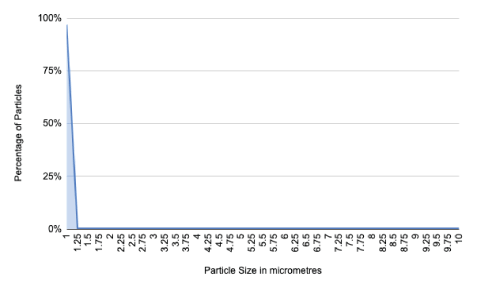
Figure 1: Graph showing particle size and percentage of total particle number.


Neha Raghava* Bojan Vidovic
Bourne hall dental practice, UK*Corresponding author: Neha Raghava, Bourne hall dental practice, UK, E-mail: neharaghava@hotmail.co.uk
In dentistry, fallow time is a period which allows for airborne pathogens to settle out of the air and mitigate the risk of airborne infection transmission to dental professionals and staff. The current recommendation is a one-hour period.
Aims and Objectives: We aim to show that air purification devices are an undervalued adjunctive measure to mitigate the risk of airborne transmission of pathogens, and so, reduce fallow time.
Methods: Using a computational fluid dynamics software, we created a virtual dental surgery and simulated a ten-minute aerosol generating procedure. We then modelled air flow in the room with no ventilation, and then in the same room with an air purifier at a throughput of 430m3h-1, and subsequently in the room with one open window providing 6 ACH and no purifier. The particles released were monitored and their behaviour and airborne time measured and collated.
Results and conclusions: The room with no ventilation had a total particle number at 600s of 4.5 million, which required 8400s to reduce by 99%. With an open window providing 6 ACH, we obtained a value of 2500s for a 99% reduction in airborne particles, and a similar peak particle volume. Conversely, when using an air purifier throughout the procedure, the peak particle number was ten times lower than the peak number without ventilation or with an open window, and after the particle injection 99% airborne particle reduction was achieved in 60s. Our findings suggest that the use of an air purifier greatly reduces the total particle volume in the air during the aerosol generating procedure, as well as the fallow period needed. The values found with 6 ACH and an open window are corroborated in other literature, which provides some validation to our testing modality. The use of air purification could greatly reduce the risk of infection transmission in a dental surgery.
Disclaimer: The contents of this publication do not constitute medical advice or guidance and should not be relied upon as such. Practices should always follow applicable laws and guidance and seek specific professional advice about their individual circumstances before taking any action based on this publication.
That which has become abundantly clear during the current pandemic is the paucity of evidence and the scarcity of literature that exists concerning the management of dental bioaerosol, and so, practicing dentistry in this climate is understandably difficult. One guideline which has come into existence to mitigate the effects of airborne transmission of the novel coronavirus is the fallow period.
The issue of fallow time and the protection of patients and dental professionals has been mired in controversy. The current Faculty of General Dental Practitioners (FGDP) [1] and Public Health England (PHE) [2] guidelines are supported by the New and Emerging Respiratory Virus Threat Advisory Group (NERVTAG) [3] and state that if an aerosol generating procedure (AGP) is conducted, it should be conducted in a room with a window and that the room should be sufficiently decontaminated prior to the next appointment. In order to allow for sufficient decontamination, a fallow period of one hour is recommended, which should allow six air changes to take place [4].
Here we aim to provide evidence of how the use of air purification can mitigate the risk of aerosol generated exposure and be used as justification for the safe reduction of fallow time within the dental profession, and aid the resumption of normal service.
Using computational fluid dynamic modelling software, we evaluated both the behaviour of aerosols and the airflow in a room with no windows or doors or air purification, and then with an air purification device in this windowless and doorless room, and compared this to a model of a room with one window and no door which provided 6 air changes per hour (ACH).
Aerosol generating procedures are not only ubiquitous in dentistry, but also integral in both routine and complex care. Aerosols are made up of particles usually less than 100 μm in diameter [5] suspended in the air, and these can sometimes be seen as the fine mist that often accompanies certain dental procedures.
At any time, expiratory activities such as breathing, talking, coughing and sneezing produce some aerosol which contains particles of varying sizes [6], and the varying sizes of these particles determines their behaviour once aerosolised. Larger particles can be thought of as ‘splatter’ and behave ballistically, falling out of the air and onto surfaces within seconds [5,7]; however, it may take up to 10 minutes for some of these to fully settle on surfaces. Those smaller than 20 μm are usually able to remain airborne for longer by virtue of their size [8]. These particles can be carried over longer distances, and, crucially, of these particles those under 10µm are considered to be within the respirable fraction [7]. This means that they are small enough to be deposited in the respiratory tract, with more penetrating particles capable of being deposited onto alveolar epithelium and thence absorbed.
The aerosol created in dental procedures at any time contains, alongside aerosolised dental materials, aerosolised components of saliva and blood as well as any pathogens contained in the patient’s sputum. This can be fairly effectively removed from the air by the use of precautions such as high volume evacuation (HVE) [9], and the risk of inhalation or deposition on exposed mucous membranes mitigated by wearing masks and visors.
However, these precautions, despite their efficacy during the procedure, are commonly removed as soon as the procedure is completed, leaving airways and mucous membranes exposed to any smaller aerosolised particles which may persist in the air long after the completion of an aerosol generating procedure [9]. In light of the novel coronavirus, this has naturally become an issue of concern.
Respiratory infections can spread either via direct or indirect routes [10] or via an air-borne route of transmission. Direct routes depend on physical contact, for example shaking hands with an infected individual should their hands be contaminated with a virus by means of coughing or sneezing into their hands. Conversely, indirect contact requires there to be a fomite such as a handrail which acts as an intermediary infected object. Airborne transmission, meanwhile, requires no physical contact between individuals [11] and takes two distinct forms: firstly, direct inhalation in proximity so larger droplets containing infectious pathogens released by an infected individual, usually over 5 μm, directly impact on a susceptible individual in proximity to the source of the spray.
The second airborne route is true aerosolised transmission; this occurs indirectly when small particles less than 5 μm in diameter, containing viable pathogens sometimes described as droplet nuclei [12], are aerosolised and can be disseminated over much longer distances and remain airborne for extended periods of time. Several respiratory pathogens such as influenza and the rhinovirus spread in this manner, alongside many other infectious diseases notably measles [13]. The time period over which pathogens remain infectious or viable largely depends on the pathogen itself and ambient conditions. However, the risk of airborne transmission lies in the potential for these droplet nuclei to be carried in the air far from the source of droplet emission, meaning that social distancing alone would be insufficient. They may also be carried to areas where personal protective equipment such as masks and visors may not be worn; even more alarmingly, there is the potential in rooms with inbuilt ventilation systems without filtration to spread the pathogens into other parts of the building [13].
Much of the preventative strategies prescribed by the government in the UK, are focused on direct and indirect transmission, i.e., the emphasis on hand washing and cleaning of surfaces, both of which are routine in dentistry. Social distancing also mitigates the effects of airborne spray transmission, although indirect airborne transmission is not mitigated by these measures. New research has shown that the airborne route of transmission of SARS-cov-2 is important-perhaps even the main mode of transmission in every day interactions [14] and it is clearly agreed that AGPs [1], such as those in dentistry which involve the fast hand piece or the ultrasonic scaler, alongside higher risk procedures carried out in hospital settings such as bronchoscopies and tracheostomies, generate large quantities of aerosol which, in an infected patient, will contain quantities of viable virus particles.
It is of clear importance, both from studies on the properties of aerosol, and the studies conducted which show the length of time over which particles remain airborne, as well the distance over which potentially infectious pathogens are disseminated [15,16], that PPE should not be removed in a room in which an aerosol generating procedure has been carried out, nor should other patients seen in the same room, until the potentially infectious aerosol has been removed or neutralised.
There are three ways, in theory, that the infection risk of this aerosol can be lessened. The most passive method is to wait for the virus to settle out of the air and onto surfaces which can be disinfected or become non viable due to the length of time elapsed outside of a host. Secondly, the use of ventilation, either mechanical or natural, to dilute and disperse infectious particles [17] would also lower infection risk, and thirdly, these particles could be actively removed from the air, i.e., via filtration.
Emerging evidence suggests that the former method takes several hours which is largely impractical in the context of a dental surgery. Ventilation forms much of the aerosol mitigation guidance currently: the underlying principle being that of increasing the air changes in the room in order to dilute and disperse any potentially infectious bioaerosol created during the course of the procedure.
Current dental guidelines created by PHE, and supported by the NERVTAG alongside the World Health Organisation (WHO) [18], suggest that once an aerosol generating procedure is performed, the room in which it was completed should not be entered without personal protective equipment (PPE) until one hour has elapsed, at which point the room can be cleaned and another patient seen.
Air changes as a way of lessening infection risk are predicated on the second method of aerosol neutralisation described above: the dilution and dispersion of infectious pathogens [17] to reduce the risk of transmission and infection. The department of health mandate 03- 01 states further that infection risk originates from the occupants of a room rather than the incoming air, and importance should be placed on dilution as a preventative strategy [19].
Air changes can be defined as the airflow in a room divided by the volume of the room and is usually expressed as a value per hour. One air change should remove 63% of pathogens in the air [17,19,20]. From this equation comes the current guidance that 6 ACH should lead to the removal of infectious aerosol from the room and allow services to be resumed.
As it stands, the length of fallow time has had serious consequences on patient care; far fewer patients can be seen in the same time period, some of whom have been unable to access care during the lockdown period, as well as serious financial ramifications for practice owners. It is therefore, an area in which scrutiny is required.
The use of air purification as a justification for the reduction of fallow time is twofold: firstly, the use of certain air purification technologies can achieve an equivalent air change when compared to the parameters set out above, i.e., at least a 63% reduction in pathogen concentration in one air change and a corresponding reduction in pathogen concentration over successive air changes [17]. Moreover, the use of some air purification technologies allows for an increase in the frequency of these equivalent air changes, so that 6 air changes may take place in far less time than an hour, reducing the safety interval between patients.
The second argument for the use of filtration and purification is that once an airborne and aerosolised pathogen is removed from the air, it follows of course that the risk of airborne transmission has been neutralised if this method of removal is effective and leak proof. If particles in the size range of these pathogens can be reliably removed from the air faster than an hour [21] it would follow that this could be a justification for the safe reduction in fallow time.
In order to be certified as a high efficiency particulate air filter, certain parameters need to be met; a true HEPA filter has a standardised efficiency of 99.97% rating for removing particles equal to 0.3 micrometres [22,23], and therefore for every ten thousand particles which pass through the filter, only three should penetrate the filter.
A study conducted by Rutala WA, et al. [21] showed that the use of a HEPA filter in a portable air purification device was able to remove particles in the most penetrating particle size of 0.3 um in the range of M. Tuberculosis in a non-hospital room in 5-6 minutes at best. In a hospital room this was achieved in 8 minutes, when compared to controls without a purifier, of 171 and 16 minutes respectively. This is a significant reduction and is of importance in this discussion as if infectious particles are removed from the air, airborne transmission is no longer a risk and they can be removed in other ways.
Miller-Leiden S, et al., also state that the use of air filtration can reduce airborne particles [24], although the values found were less dramatic (30-90% in an airflow of only 2 air changes per hour) and found this to be an effective adjunct in the control of tuberculosis. The caveat they raise is that in non-perfectly mixed air, these filters may be less effective; however, this caveat is of little import in this discussion as the air change theory using any form of dilution is also predicated on perfect mixing of air [17].
NHS Scotland guidance does mention the use of air purification as a mitigating factor in fallow time calculations, but it suggests a 0.5 efficiency factor to allow for this issue of recirculation and imperfect mixing times [25]. Even when this efficiency factor is taken into account, it is clear that the performance of an air purifier is far more reliable than that of natural ventilation. This is because natural ventilation relies, of course, on several different factors- such as ambient and atmospheric conditions, and may at times be far lower than the 6 ACH value needed to make an hour’s fallow period valid.
In addition to this, studies which specifically measured the penetration of virus particles through HEPA filters found reassuring viral retention values of between 99.996% and 99.97% [26,27], although this did vary with environmental conditions; for example, when considering efficacy in infection control it is clear that other factors such as airflow and humidity are of great importance [23,26,27]. However, if we operate on the assumption that natural ventilation is being used as is the current standard of practice, the use of HEPA filtration has been shown to be an effective method to create an adjunctive reduction in airborne particle concentration and a reduction in fallow time.
Indeed, the HTM-03-0119 document produced by the department of health states that HEPA filters are required in ultra clean systems such as theatres and aseptic rooms: HEPA filters are expensive. Therefore, their use should be kept to a minimum. Applications requiring HEPA filters include the air supply to aseptic suites in manufacturing pharmacies and the discharges from microbiological safety cabinets [19].
Given the current risk of transmission in non ultra-clean systems, the cost of HEPA filtration may seem negligible in comparison to the effects of the creation of a safer environment and the reduction of fallow time.
This is not to say that all purification units are suitable and as stated in the same memorandum, they must be replaceable, with leak proof seals, and equipped with a safe method of disinfection and replacement so as not to create another source of infection within the dental practice [19].
It is of brief significance to mention other technologies such as the use of ultraviolet C (UVC) radiation alongside HEPA filtration, as UVC has been used to disinfect equip-ment which may have come into contact with viruses such as SARS-CoV-2 [28,29], however, little literature exists on how effective commercial air purification devices are at delivering a threshold dose.
We used openFOAM, a computational fluid dynamics software in order to model aerosol behaviour. A room of dimension 3 × 3 × 2.4 m3 was modelled, in which we placed a dental chair and operator, in order to recreate, as closely as possible, the conditions in a dental surgery in the UK.
In order to make the simulation as accurate as possible we also modelled heat released by the operator, patient and overhead light in both simulations. This is an important point to note, as in a dental practice the air will never truly be static due to some minimal air flow created by the effects of heat sources and buoyancy. Our simulations did not contain doors as during aerosol generating procedures it is currently required that doors be shut to prevent aerosol leaving the room.
The air purification unit used in our simulation is the currently commercially available MedicAir unit which combines HEPA filtration with UVC radiation and a carbon filter. It measures 0.8m in height and 0.3m in diameter and was exactly replicated in the simulation. including the 5000 inlet holes for air suction. During our simulation the purifier was operated at a throughput of 430 m3 h-1, which is an air flow rate at the higher end of the throughput offered by commercially available air purifiers.
We then decided to recreate an ultrasonic scaling appointment without the use of HVE as a worst-case scenario [30] hence our choice of a flow rate of 17ml min-1 as this is the typical flow rate of an ultrasonic scaler. The duration of this flow was 10 minutes, and the total particle number released was 4.5 million.
We chose the 10 minute exposure in order to create results comparable with other experiments in the literature on this subject, and also as this is a figure quoted in NHS Scotland guidance [15,16,25].
In order to further mimic the dental appointment, we used data on the typical distribution of particle sizes generated during dental procedures, most of which were around 1 micrometre in diameter [5,6,8]. In order to determine the total particle mass we used the density of water, of which most of dental aerosol is composed, in order to more accurately model particle behaviour, which is determined largely by their size [8]. Another benefit of using particles of these sizes is that they all fall within what is commonly thought of as the respirable fraction [7]. This is useful modelling as the novel coronavirus appears to target ACE-2 receptors found in the lungs, so the highest risk of transmission will be from particles in this size range. Therefore, this model will serve as a simulation of a worst-case scenario in which all the particles in the aerosol are infective and capable of penetrating the lower respiratory tract. It should be noted of course that usually the viral content of an aerosol is taken to be the same as the sputum concentration, which is a far lower figure (Figure 1).

Figure 1: Graph showing particle size and percentage of total particle number.
In order to accurately simulate the time particles remain airborne, the simulation with no ventilation was done using first a steady-state flow solution which was obtained mathematically in order to account for buoyancy effects. Lagrangian particle tracking was also used. Particles were injected from the patient’s simulated mouth for 10 minutes and their number was monitored. This was then mirrored in the simulation in which the air purifier was present.
Particles are modelled as affected by gravity and drag which is modelled as sphere drag and, of the particles in the study, all were 10 μm and below.
Using the parameters of the size of the room, heat sources and adiabatic objects and isotherms, we then created a flow field for both the room with an air purifier and the room without, which showed us the patterns and directions of flow, as well as the velocity.
We then created and ran simulations in which we injected the particles for ten minutes continuously at the aforementioned flow rate. These showed the behaviour of the particles during the AGP and continued the simulation until all the particles were cleared with an air purifier in the room. There were no open windows or doors in the simulation.
Following this simulation, we used the same typical dental surgery created without any open windows or doors and evaluated the aerosol behaviour without an air purification device in order to evaluate the time taken for the aerosol particles to settle out of the air.
Our third simulation was based on the same room without an air purification device, but with an open window. This was an important scenario to model due to the emphasis in guidance documents on the open window as a method by which to dilute and disperse bioaerosol in a dental surgery. The parameters remained the same as in the above simulations, and the AGP was simulated for the same length of time. A difficulty with modelling this simulation is mirrored in the evident flaws in the use of natural ventilation, i.e., that there is huge variation based on external conditions which vastly impacts the ACH in a room with an open window.
Another pressing reason to model this open window scenario is the paucity of studies undertaken on this, despite the volume of guidance which recommends it. One study recently undertaken by Haigh, Vasant and O’Hooley [31] attempted to display the effects of an open window on particle removal in another mannequin study; however, they were unable to definitively quantify the effect this had, although they did conclude that it reduced airborne particle volume. Their study also did not include particles below 0.3 micrometres, which is a disadvantage given that this is the size of particle into which viruses fall.
Given this, we decided to validate our methodology by setting the ACH to 6, which is the value stated in the CDC and PHE guidelines. We also decided to use the area of a typical window, 0.65 m × 0.89 m, as our opening.
Therefore, using the WHO [18] equation for calculating air changes using a window:
0.65 × wind speed m/s × smallest opening area m3 × 3600 s/h room volume m3.
We obtained a value for the wind speed required to provide 6 ACH for a room of the size used in our simulation and were able to create a flow field and then run this simulation also (Figures 2-7).
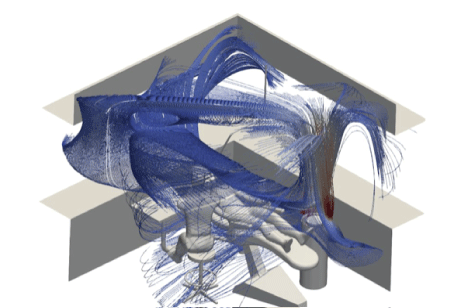
Figure 2: Flow field with air purification.
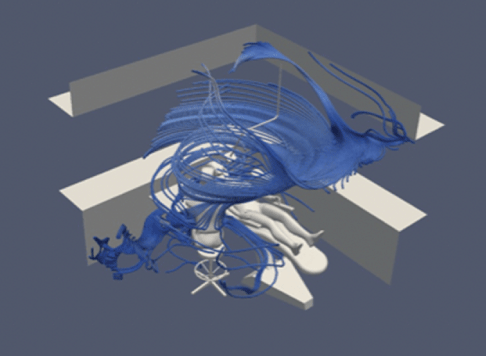
Figure 3: Flow field without air purification.
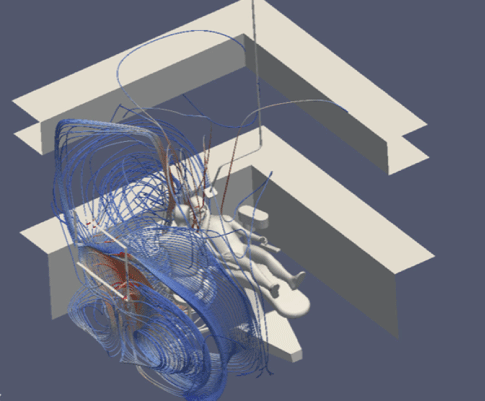
Figure 4: Flow field with one open window and 6 ACH.
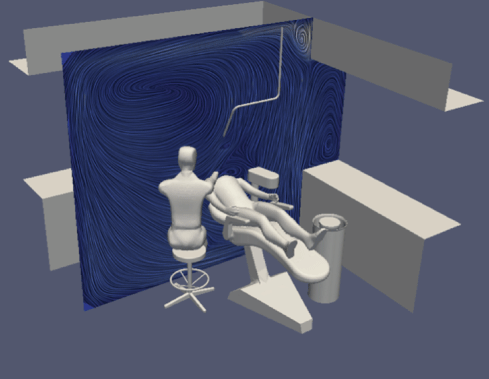
Figure 5: Flow velocity and direction with air purifier.
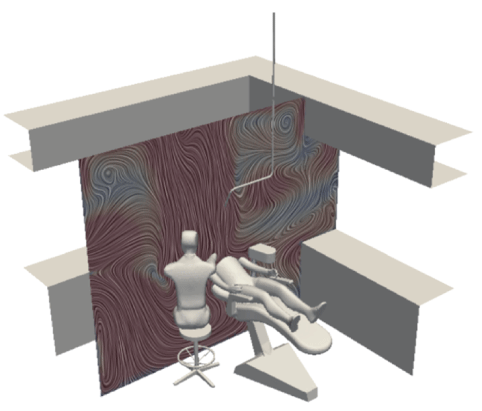
Figure 6: Flow velocity and direction without ventilation.
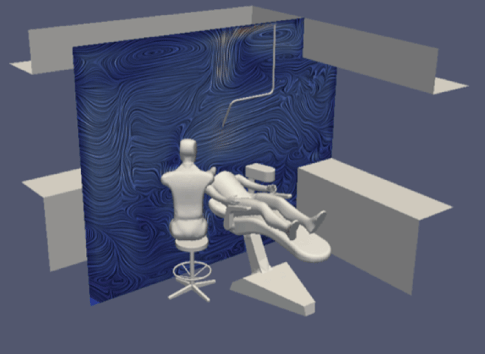
Figure 7: Flow velocity and direction with an open window.
Subsequently we collated the data for all three simulations, and this gave us figures 8-11, as discussed below.
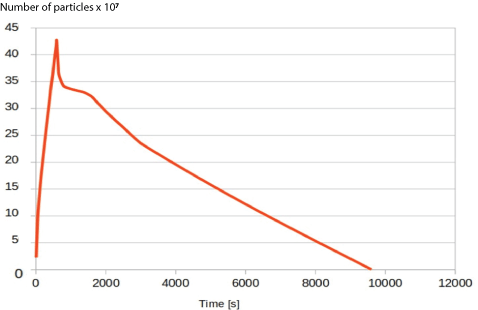
Figure 8: Graph showing the change in airborne particle number over time in the room with no ventilation.
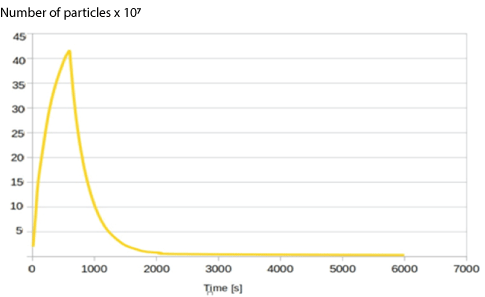
Figure 9: Graph showing the change in airborne particle number over time with an open window providing 6 ACH.
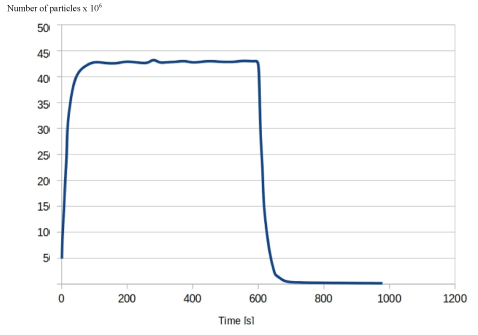
Figure 10: Graph showing the change in the number of airborne particles over time in a room with an air purification device.
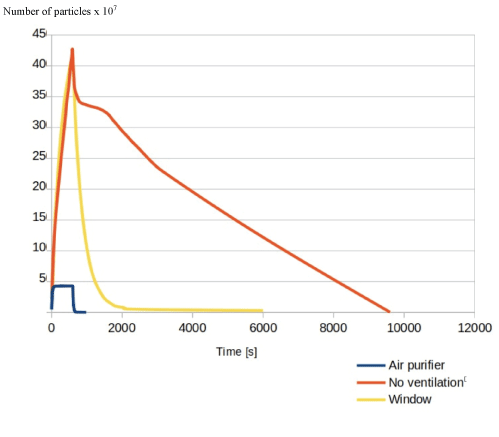
Figure 11: Graph showing the change in airborne particle number over time in all three simulations.
Firstly, using the parameters set out above, we mapped a flow field for all simulations. This showed us the velocity and direction of airflow with and without air purification, and with natural ventilation.
After this, we then modelled the AGP by continuously injecting aerosol particles into thesystem for ten minutes at a flow rate of 17ml min-1 at the sizes and volume detailed in the methodology, both in the room with no ventilation and air purification, and the room containing the air purifier, as well as in the room with natural ventilation.
The graph in figure 8 shows the results of the simulation with no ventilation. At 600 s, the particle injection stops, and the peak particle number reached is around 4.5 million, and the graph can be extrapolated to give a settling time for 99% of the particles at 8400 minutes, and to 99.9% at 9000s.
Figure 9, with an open window shows that the use of natural ventilation, provided there is sufficient wind speed throughout to provide 6 ACH, provides a significant reduction in fallow time, with a drop of 99% at 2500s, and 99.9% at 6400s based on linear approximation. The peak particle number reached was similar to the case with no ventilation.
Conversely, figure 10 shows the same figures for the simulation in which the air purifier was present and operating at a throughput of 430 m3h-1. With the use of an air purifier, not only is the time taken to clear 99% of airborne particles 60 seconds after particle injection stops, but the total particle number is 450,000, which is a tenfold decrease from both graphs above. The particle number drops further by 104s post AGP, at which point 99.9% of particles are removed from the air.
Interestingly, the total time taken for a 99% reduction in airborne particle number in the model with no ventilation is far lower than figures for 1 and similar to those values found for 2 air changes (Figure 11).
The current guidance on the fallow period is based on the use of a mathematical model, and using the formula which relates the purging of airborne contaminants to air changes to reduction in pathogen concentration:
t2 − t1 = –ln(C2/C1) Q/V × 60, (1)
with t1 = 0
where: t1 = initial time point in minutes,
t2 = final time point in minutes,
C1 = initial concentration of contaminant,
C2 = final concentration of contaminant,
C2 / C1 = 1 – (removal efficiency / 100),
Q = air flow rate in cubic feet/hour,
V = room volume in cubic feet,
Q/V = ACH [4]
We can obtain values of 138 minutes to clear 99% of airborne contaminants with 2 ACH, 69 minutes with 4 ACH, 46 minutes for 6+ and 35 for 8 ACH.
For n number of air changes the corresponding reduction of 0.37n will then occur [4,17]. With 5 air changes, 99% of airborne contaminants in a room should be removed, and in 6 all contaminants should be removed [17]. Increasing the number of air changes in a room per hour, therefore, reduces the time taken to remove these contaminants: for example in surgical theatres the accepted standard is 12 air changes per hour, which would mean 25 minutes of fallow time would be sufficient [17,18], and an ultra clean system, which usually has 25 air changes per hour, would need even less fallow time [19].
The current guidance for achieving this is natural ventilation, i.e., undertaking any aerosol generating procedures in a room with a window and not entering the room without respiratory protection until an hour has passed.
This recommendation is flawed on several counts; firstly, this does not take into account the duration of the AGP, which affects the total aerosol particle volume released into the air. Secondly it is predicated on the value of 6 ACH in a room with an open window, but studies which have measured air changes in a residential building with an open window a similar model of a neutral pressure room have obtained values far lower than 6: in the region of 0.45 and 1.9 ACH with one open window, and 0.47 and 3.07 with multiple. This is a far lower value and would require a much longer fallow period than an hour (Figure 12).
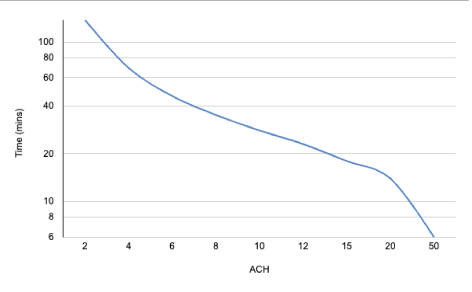
Figure 12: Graph showing the relationship between ACH and time taken to remove 99% of airborne contaminants [4].
NHS Scotland [25] recently released further guidance in terms of ventilation and air changes which takes into account the length of the AGP when deciding on a fallow period, based on a 3 × 3 × 4m room. This guidance recommends a minimum fallow period of 10 minutes regardless of ACH to allow time for particles to settle. An advantage of this guidance is the allowance in the calculation for the use of mitigating factors such as the use of HVE and rubber dams- and a clear, quantified corresponding reduction in fallow time based on which mitigation strategy is used.
This is much more reassuring than the FGDP [1] guidance which suggests that this fallow period can be reduced based on the practitioner’s individual assessment of aerosol generated exposure risk, as placing the responsibility for reducing fallow time which could have catastrophic effects on staff and patient safety if misjudged on the practitioner can only add to the immense stress [32] several of our profession have been under since the beginning of the pandemic (Figure 13).
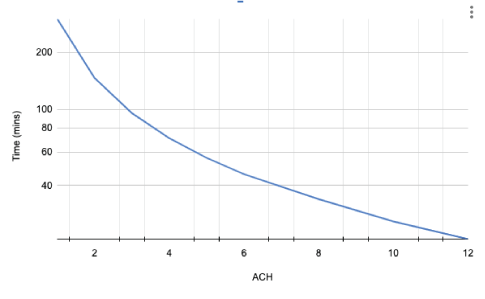
Figure 13: Graph showing the relationship between the length of time taken for the clearance 99% of airborne particles released in a 10-minute AGP and ACH, with no mitigation [25].
In the FGDP guidelines the use of HEPA filters is mentioned as a weakly evidenced adjunctive measure [1], however, we would argue that following the same air change rationale presented by governing bodies, the use of air filtration often included in commercial air purification units is a well evidenced intervention that can greatly reduce the time over which infectious pathogens remain aerosolised, and therefore reduce the risk of inhalation of pathogens for both patients and dental professionals when used alongside natural ventilation.
Conversely, the CDC recently amended their earlier guidance to recommend. Considering the addition of portable solutions (e.g., portable HEPA filtration units) to augment air quality in areas when permanent air-handling systems are not a feasible option [4].
This can be taken to mean those rooms without windows, but perhaps also rooms with windows, if ACH with natural ventilation alone is insufficient or unverified.
The NHS Scotland guidelines [25] are clearer on the use of air purification, or ‘air scrubbers’, in that they suggest that air purifiers be given an efficiency factor of 0.5 in order to allow for recirculation of air and imperfect mixing. This means that the maximum throughput of the air purifier should be halved, and the volume of the room divided by this in order to give an ACH value. These guidelines also suggest that AGPs can be undertaken in rooms with mechanical ventilation without natural ventilation if the number of ACH can be verified.
This guidance on the use of mechanical ventilation in order to create ACH lends credibility to the use of air purification as a way of reducing both transmission risk and reducing the fallow period.
The results of our simulations, shown especially clearly in figure 11, evidence that based on this study, there is a drastic reduction in the time taken to clear 99.9% of air borne particles from the air, when compared to the open window model.
Moreover, our study was also able to show that having the purification unit running throughout the simulated AGP meant that the overall peak particle concentration was ten times lower than that in the open window simulation and the model with no ventilation. We attribute this to the active nature of air purification in creating air flow when compared to the passive open window model.
This means that not only does the fallow period reduce with the use of this adjunctive measure, the overall aerosol generated exposure of dental professionals and patients to potentially infectious aerosol is reduced by the use of purification throughout AGPs.
A review of the literature showed that there have been few studies on the dissemination of bioaerosol in dental surgeries, however, Allison JR, et al., [15] conducted a study on dissemination of bioaerosol in dentistry, in which an anterior crown preparation was performed on a mannequin in a room with 6.5 ACH, and closed windows and doors. The water used as a coolant in the fast handpiece was contaminated with fluorescein, and after the 10 minute AGP, the resultant splatter settled onto filter paper which was placed at different distances from the site of the AGP. The filter paper was replaced and evaluated at 30 and 60 minute intervals. This paper found that in a room with 6.5 ACH, settling of airborne particles took place within 30 minutes, and the recommendation based on this was that the fallow period could be reduced to 30 minutes.
Veena HR, et al., conducted a similar study in 2015 [16] in which ultrasonic scaling was per- formed for 15 minutes, again using water in which fluorescein was dissolved, and subsequently deposited on filter paper at different distances from the mannequin. Immediately after the scaling was completed, the filter papers were replaced with new ones, every 30 minutes for 90 minutes. Their results showed that the aerosol remained in the air for 30 minutes after scaling; unfortunately they did not detail the number of air changes in the room in which the experiment was conducted.
Both of these experiments found that the maximum contamination was within 2 metres of the site of the AGP. However, Allison JR, et al., [15] study also found some contamination 4 metres away from the patient when not using a HVE, which was the furthest distance they measured.
The limitations of these studies lie in their use of mannequins, which do not mimic the effect of the human body on fluid dynamics, and their inability to continuously monitor airborne particle number. The use of filter paper is also, perhaps, an insufficiently sensitive quantification modality. There was also some evidence of secondary sedimentation in Allison et al’s study at 60 minutes which was not fully explained [15].
We chose to use this 10-minute AGP duration in our modelling in order to create comparable results with Allison JR, et al., [15] study when evaluating the use of air purification in removing aerosolised particles from the air, both in order to validate our own methodology, and to provide a comparison when particle number was continuously modelled.
The time taken for the open window simulation with 6 ACH to reduce particle count by 99% in our simulations, is lower than the CDC and NHS Scotland values [25] but fairly consistent with the 30-minute value with 6.5 ACH found in Allison JR, et al., paper [15]; we suggest that the difference in time taken for the particle number to fall is largely due to the smaller room size used in our simulation. This corroboration with Allison JR, et al., study also functions to validate the physics of the external and internal environment in the simulation.
We propose that the difference in values found for the number of airborne particles to decrease by 99% with no ventilation, when compared to our simulation of a room without ventilation, is due to the detailed nature of our simulations. Particles may settle out of the air onto surfaces even in a room with no ventilation much faster than predicted when buoyancy and slight air movement is taken into account, as is the case when heat sources are present in the room, just as they would be in a dental surgery.
The limitations of our study are largely based on its simulated nature, which may not exactly mirror in vivo results; however, in order to mitigate the effects of this, we endeavoured to match the simulation as closely as possible with atmospheric conditions. Furthermore, as other studies, such as those conducted by Allison JR, et al., [15] and Veena Hr, et al., [16], do not contain a method in which to evaluate and monitor the change in airborne particle number over time, we propose that modelling these conditions in simulations such as these helps to fill an important gap in our knowledge.
Another limitation is that the simulations were only run for 6000 seconds, and therefore the values for a 99.9% reduction for both the open window and the no ventilation simulation were linearly extrapolated, and we would encourage any further modelling to run for longer. It is also possible that an AGP of the same duration could release a total particle number higher or lower than the value used in our study.Moreover, there is very little literature which studies the size distribution of particles in dental aerosol, and we would welcome further research in this area.
A point to note is that in order to provide 6 ACH, our window simulation required a constant external wind speed of 0.096 m/s, with a window area of 0.65 × 0.89. This value is often met by the average wind speed in the UK. However, the direction and angulation as well as internal and external pressures and temperatures should also always be favourable, which were fixed in our simulation but are uncontrollable in reality.
Using the same models upon which the air change guidance provided by the CDC [4] and other governing bodies provide is based, it is clear that if air changes can be effectively increased the fallow period can be reduced. However, outside of a hospital environment, installing mechanical methods of increasing airflow and changing air pressures is difficult and occasionally prohibitively expensive, if not impossible, in the current configuration of many dental surgeries.
The use of portable air purification involving HEPA filtration is a reliable adjunctive measure that can be used to reduce fallow time and improve safety in the dental environment, and this has recently been validated by NHS Scotland [25].
This can be evidenced using the air flow modelling studies we undertook, which show that based on the fluid dynamics of a room of average size with this air purification unit, the total time to clear particles from the air is drastically lower than in a room without an air purification device. In our simulation, with the use of an air purifier, 99.9% of airborne particles were removed from the air in 1 minute and 44 seconds post AGP.
Our results also showed that the mechanism of action of this air purification device was twofold in that airborne particles were either drawn into the device and filtered out of the air, or the fluid dynamics of the room created by the air purification device force airborne particles to settle onto surfaces which can easily be disinfected, removing any pathogens present.
Importantly, we were able to show that by using the air purification device throughout aerosol generation, the peak particle number during the procedure was ten times lower than in the room without an air purification device, or a room with an open window, which would greatly reduce the risk of airborne infection transmission.
This work was commissioned by Bryant Medical Ltd. The funders had no role in study design, data collection or analysis. Bryant Medical Ltd was involved in the preparation of the manuscript and the decision to publish.
Based on the nature of the problem, kOmegaSST turbulence was chosen for the analysis. It has been used on many similar cases with good agreement with experimental results. Kinematic viscosity values for air at 22 degrees Celsius is used (Figures 14-17).
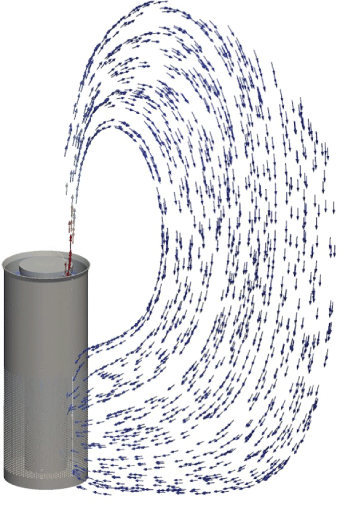
Figure 14: Flow field air circulation.
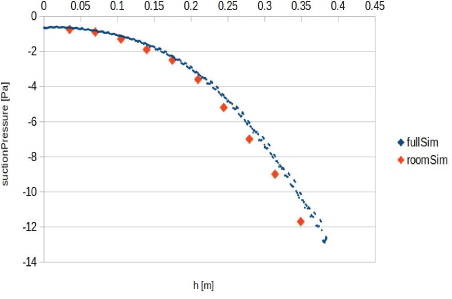
Figure 15: Flow field in holes vicinity (left); air circulation through the device. This analysis helps to get a proper value for suction pressure when modelling the full room. Suction pressure is set on the room analysis cases based on this data. The air purification device in our simulation was placed 0.5m left of the patient, in line with their feet.
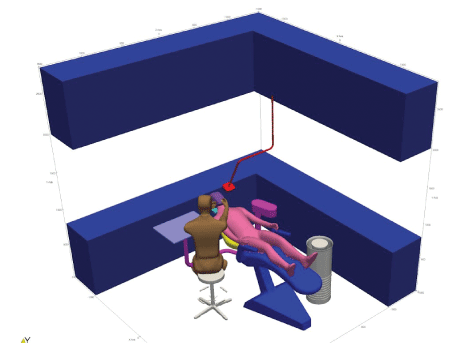
Figure 16: Geometry for room analysis.
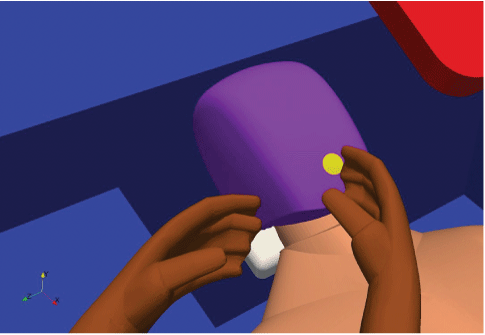
Figure 17: Geometry for room analysis, patient mouth detail. In order to analyse the particle injection a circular mouth was modelled, and it is from this that the particles were injected, mimicking the oral cavity.
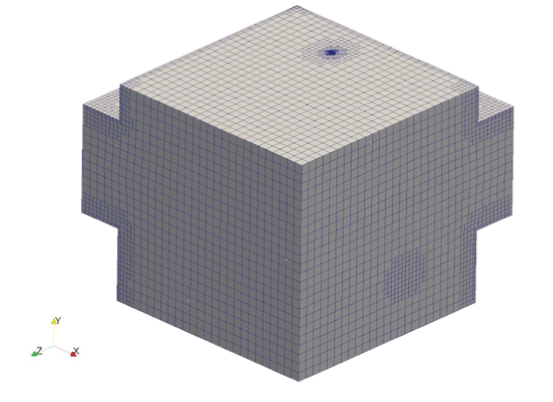
Figure 18: Mesh for room analysis.
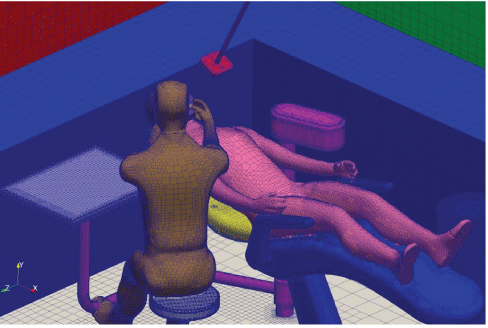
Figure 19: Mesh surfaces.
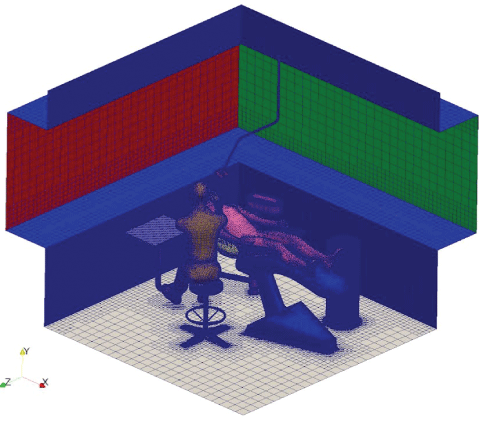
Figure 20: Mesh surfaces, detail.
Figure 21: Mesh for room analysis, sectional.
Figure 22: Mesh for room analysis, section detail.
Figure 23: Mesh for room analysis, double section.
Figure 24: Mesh for room analysis, double section detail.
| Patch Name | Cell size min [m] | Cell size max [m] |
| Dentair device | 0.003125 | 0.00078125 |
| Inlet | 0.003125 | 0.00078125 |
| Outlet | 0.00078125 | 0.00078125 |
| Patient head | 0.0125 | 0.00625 |
| Patient body | 0.025 | 0.00625 |
| Dental chair | 0.025 | 0.003125 |
| Stool | 0.003125 | 0.00625 |
| Lamp | 0.00625 | 0.003125 |
| Cupboards | 0.1 | 0.05 |
| Boundary Conditions | Values |
| Pressure | Fixed-101325 Pa |
| Velocity | No slip |
| Temperature | Adiabatic Surfaces for walls and furniture |
| Patient and dentist-36.5 degrees Celsius | |
| Dental lamp-57 degrees Celsius |
| Air Purification Device Conditions | Values |
| Pressure | Fixed-101325 Pa |
| Velocity | Flow rate 430 m3h-1 |
| Temperature | Adiabatic surface |
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Raghava N, Vidovic B (2021) Using Computational Fluid Dynamics to Evaluate the Role of Air Purification in Reducing Fallow Time in Dentistry. Int J Dent Oral Health 7(4): dx.doi.org/10.16966/2378-7090.362
Copyright: ©2021 Raghava N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access