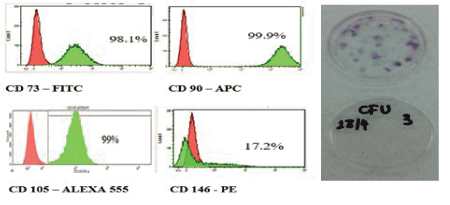
Figure 1: A-Graphs showing the intensity of fluorescence using flow cytometry of GMSC surface markers.
B-A representative image of colony-forming unit assay.


Al Bahrawy M1* Ahmed Gamal2 Khaled A Ghaffar3 Vincent Iacono4
1Department of Periodontology, School of Dentistry, Ain Shams University, Cairo, Egypt*Corresponding author: Al Bahrawy M, Department of Periodontology, School of Dentistry, Ain Shams University, Cairo, Egypt, E-mail: bahrawy21@hotmail.com
Background: Cell migration through micro perforated membranes might help in managing the periodontal defect isolation from the surrounding regenerative elements, a problem caused by guided tissue regeneration using occlusive membrane. Macro perforation of a membrane affects its mechanical properties and eliminates its role as a barrier against gingival epithelium and extracellular matrix components.
Materials and Methods: Human Gingival mesenchymal stem cells (GMSCs) were seeded on the upper chambers of collagen-coated polytetrafluoroethylene (PTFE) transwells with readymade pore diameters of 0.4 and 3 microns and polycarbonic acid transwells with readymade pore diameter of 8 microns. Fetal Bovine Serum (FBS) was added to the culturing media in the lower chambers versus plain media in the control group. Migrating cells were counted in the lower compartment. Scanning electron microscopic imaging of the lower surface of the perforated transwell membranes was obtained.
Results: Human Gingival mesenchymal stem cells migrated more significantly in FBS chemotaxis groups compared to the control group. The 8-micron perforated membrane group showed statistically significant more cell migration compared to the 3-and 0.4-micron groups. Scanning electron microscope images confirmed cell migration through the perforations.
Conclusion: The results of this study demonstrated that membrane microperforations of 0.4, 3, and 8 microns are suitable pore diameters for Human gingival mesenchymal stem cell migration to chemotactic media and are occlusive for cell migration in negative control, without affecting membrane mechanical or occlusive properties, which can be used to develop GTR membrane with selective cell migration ability.
Guided tissue membrane; Gingival mesenchymal stem cells; Periodontal regeneration; Guided tissue regeneration; Periodontal pockets
Full regeneration of periodontal apparatus is difficult to achieve despite the inherent regeneration ability of the periodontium [1]. Conventional periodontal therapy most often results in repair with granulation tissue and long junctional epithelium, which means loss of function [2].
In 2009 Zhang, et al. isolated and characterized mesenchymal stem cells (MSCs) from human gingival tissue [3]. Tomar, et al. compared human gingiva-derived MSCs (GMSCs) to bone marrow-derived cells and demonstrated their advantages in many respects [4]. The existence of such cells with multipotent capacity in the gingival connective tissue urges an essential modification in guided tissue regeneration (GTR) membrane regarding its occlusive nature which deprives the periodontal wound from such cells [5].
Mardas, et al. [6] compared bone formation in permeable Teflon membrane capsule with 300-micron perforations to control occlusive membrane capsule containing demineralized freeze-dried bone allograft (DBM), where similar amounts of bone were formed in the permeable and occlusive capsules. The researchers concluded that the invasion of undifferentiated mesenchymal cells into the periodontal wound area was unnecessary.
Clinically, Gamal and Iacono [7-9] compared traditional occlusive barrier membrane to perforated collagen membrane. A statistically significant reduction in pocket depth and increase in clinical attachment and clinical bone level for both membranes were found, with more improvement in the perforated membrane group than the occlusive membrane group.
The optimal perforation diameter that selectively allows mesenchymal stem cell migration through GTR membrane without jeopardizing the membrane occlusive nature has not been explored yet. In this study, three perforation diameters were experimented, with two of them never being evaluated before for GMSCs locomotion dynamics through perforated membranes.
In a study by Bhattacharya, et al. oral keratinocytes showed the ability to migrate through 8-micron perforated polycarbonate membrane [10]. This data would suggest that membranes with perforations larger than 8 microns might allow the invasion of the periodontal defect by the gingival epithelium.
To the best of our knowledge, this study would be the first in scientific literature to prove the ability of GMSCs to migrate through pore diameters as small as 3 µm and 0.4 µm in response to chemoattractant and to demonstrate that 8 µm perforated membrane is occlusive for cell migration in absence of chemotactic factor. This finding throws light on the possibility of using suitable chemotactic agents to allow the migration of certain cell lines from the surrounding tissues to the periodontal defect.
The data obtained from this study would be used to develop the function of GTR membrane from being only a mechanical barrier to a more tissue-engineering suited device used to filter cells in cell homing technologies.
The main objective is to investigate the migration potential of GMSCs through micro-diameter pores to chemotactic factor versus plain media negative control, as a basis for further studies on the use of different chemotactic factors to attract special cell lines with specific markers to the periodontal wound.
Gingival tissue biopsies (3 × 2 × 2 mm) were obtained from three healthy adult patients (34, 30, and 43 years old). Healthy gingiva is composed of epithelium and underlying connective tissue, harvested during crown lengthening procedure at a periodontal care clinic at Stony Brook University, Long Island, NY, USA.
The study was approved by the Committees of Research Involving Human Subjects at Stony Brook University and Ain Shams University approval number [575741-1] on 10/8/2014 in both universities. Verbal consent was obtained from the participating subjects; they were informed about using their discarded tissues in stem cell laboratory studies.
Gingival samples were transported to a bioengineering and stem cell laboratory in stony brook university NY. In an alpha-modified Eagle’s medium (α-MEM) enriched with 10% Fetal Bovine Serum (FBS) and 1% Penicillin/Streptomycin and Amphotericin in accordance with culturing technique reported by Mitrano, et al. [11].
The gingival tissue samples were washed in phosphate-buffered saline (PBS) many times, and epithelium was removed using a surgical blade. The connective tissue specimen was sliced and minced into small pieces using surgical blade number 15 measured approximately 1 × 2 mm. The minced connective tissue specimens were collected in 10 mL tubes and then digested with McCoy’s modified media: in 2 mg/mL Dispase II (Sigma-Aldrich, St. Louis, USA) in 4°C overnight and then in 2 mg/mL collagenase IV (Sigma-Aldrich, St. Louis, USA) for 40 minutes in 4°C [12]. The digestion reaction was inhibited by adding culture media composed of Dulbecco’s MEM enriched with 10% FBS (Gibco, Invitrogen Life Technologies, MA, USA), Penicillin/ Streptomycin (G100 units/mL, 100 µg/mL), and Amphotericin (Fungizone 0.25 µg/mL).
The digested tissue was filtrated through a 40 μm cell strainer (Sigma-Aldrich, St. Louis, USA) to obtain single-cell suspensions and then centrifuged in 10 mL tubes for 1200 rpm 200 RPC for 10 minutes. The obtained cell pellets were suspended in a culture medium by successive pipetting and then collected and seeded at a concentration of 60 cells/cm2 in 10cm tissue culture dishes for the selection of single-cell-derived colonies in α-MEM 1X (Gibco, Invitrogen Life Technologies) supplemented with 10% FBS (Hyclone Laboratories, Inc., Logan, UT, USA), 50 U/ml Penicillin G with 50 μg/ml Streptomycin, and 2.5 μg/ml Amphotericin B (Fungizone) at a humidified atmosphere 37°C, 5% CO2 . Twenty-four hours later, cells were washed and then fresh medium was added; cells were fed every 3 days until the end of the experiment.
Gingival mesenchymal stem cells have been monitored during cell proliferation; subculture was done when primary cell culture reached 90% confluence, which was labeled passage 0, and later passages were labeled accordingly.
The proliferation capacity was assessed by population doubling assay [13]; GMSCs were seeded at 5 × 103 cells/cm2 in 24-well plates, expanded to approximately 90% confluence, and detached with 0.05% trypsin/EDTA; then cells were counted. GMSCs were reseeded at 5 × 103 cells/cm2 into another well of a 24-well plate and cultured until in vitro cellular senescence is reached. Cell counts were performed at each passage, and population doublings were calculated using the following formula: log2 final cell number/log2 seeding cell number.
The final population doubling values for the GMSCs were represented as the sum of population doubling values obtained at each passage for three successive passages.
The fifth-passage gingival mesenchymal stem cells (GMSCs) were observed under inverted light microscope for cell aggregates with colonies of more than 50 cells. The morphometric analysis was done for cell shape and measurements, where the first single cell showed plastic adherence detected after 4 days and irregular spindle shape like fibroblast morphology after 7 days.
The colony-forming unit experiment was done in triplicate [14]. Freshly digested gingival tissue was seeded at 1000 cells per dish in P10 plates, cultured in α-MEM with 10% FBS, and supplemented in a humidified atmosphere (37°C, 5% CO2 ). The culture medium was changed twice per week. After 14 days, colonies were washed twice with Phosphate Buffered Saline (PBS), then fixed with 4% paraformaldehyde solution, and stained with hematoxylin and eosin for counting colonies.
Approximately 1 × 105 cells were incubated with 2 μg/ml fluorescein isothiocyanate- (FITC-) conjugated mouse monoclonal antibodies specific for human CD73 and its isotype, allophycocyanin- (APC-) conjugated mouse monoclonal antibodies for CD90 and its isotype, phycoerythrin- (PE-) conjugated mouse monoclonal antibodies for CD146 and its isotype (BD Pharmingen, San Diego CA, USA), and Alexa 555 goat anti-mouse for primary unconjugated mouse monoclonal antibodies against CD105 (DAKO, Carpinteria, CA, USA); its control was Alexa 555 goat anti-mouse without primary mouse antibodies. Hematopoietic stem cell markers and mouse monoclonal antibodies were used against CD14, CD34, and CD45 (eBioscience, San Diego, CA, USA) for half an hour in 4°C. After being washed by centrifugation and re-suspension twice, cells were subjected to flow cytometry analysis using BD LSRFortessa cell analyzer (BD Biosciences) at Stony Brook University Hospital, NY, USA.
Gingival mesenchymal stem cells (GMSCs) were seeded at 8 × 103 cells per cm2 in six-well plates and cultured in osteogenic media [15], an adipogenic medium [16], and a chondrogenic medium (Gibco Invitrogen, Carlsbad, CA, USA). For During the period of 28 days, media were changed twice weekly; then wells were washed twice with PBS, and cells were fixed with 4% paraformaldehyde. Mineral deposition was identified by incubation with 2% Alizarin Red, oil droplets with Oil Red O, and cartilage glycoproteins with Alcian Blue (Sigma Chemicals, St. Louis, MO, USA).
Cells were seeded in spectrophotometer tube with 500 µl α-MEM (Gibco Invitrogen, Carlsbad, CA, USA) enriched with 10% FBS (Hyclone Laboratories, Inc., Logan, UT, USA). The control group received cell-free media. Tubes were incubated with 100 µl MTT reagent (Trevigen Inc., Gaithersburg, MD, USA) in 37°C for 4 hours until purple crystals precipitated; then media were aspirated, and 1000 µl DMSO was added to each tube to solubilize the formazan dye. Finally, the extent of MTT reduction to formazan was quantified by absorbance using a spectrophotometer at wavelength of 595 nm, which is directly proportional to the number of living cells within the specimen; the experiment was done in triplicate. The MTT score was calculated by subtracting the spectrophotometer reading of MTT staining of GMSCs from the control readings.
Cell migration assay was performed in a transwell chemotaxis chamber [17], and the cells were divided into three groups. The first group included perforated polycarbonate membranes with pore diameter of 8 µm (6.5 mm in diameter; Corning Life Sciences, Corning, NY, USA). The second and third groups included perforated collagencoated PTFE membrane (12 mm in diameter; Corning Life Sciences, Corning, NY, USA) with pore diameters of 3 µm and 0.4 µm as inserts applied in 12-well polystyrene plates. Cells were harvested using PBS with 0.05% trypsin/EDTA and re suspended in serum-free α MEM. The transwell inserts in the upper chamber were loaded with 10,000 cells in serum-free α-MEM; the lower chamber content differed according to the group type: in the control group, FBS-free α-MEM was used in the lower compartment with no chemotactic factor in the chemotaxis groups, α-MEM supplemented with 10% Fetal Bovine Serum (Hyclone Laboratories, Inc., Logan, UT, USA) was used as a chemotactic factor. The cultured cells on the inserts plates were incubated in humidified atmosphere (37°C, 5% CO2 ) for 24 hours; then the media in the upper and lower chambers were aspirated, inserts were washed twice with PBS, and the cells on the upper side of the membrane were removed with a cotton swab. Cells that migrated to the lower side were fixed with 4% paraformaldehyde (PFA) for 2 minutes, washed twice with PBS, permeabilized with 100% methanol for 20 minutes, stained with crystal violet 1% in 80% ethyl alcohol (Sigma Aldrich, St. Louis, MO, USA), and washed again twice with PBS.
The transwell membrane was cut off to be removed from the inserts, and the lower side of the membranes was marked with a pen for identification, with a total of six membranes for each group. Migrating cells were counted using inverted light microscopy at 40X magnification at 5 different representative fields, and the mean of each insert was calculated for statistical analysis; the experiment was done in triplicate.
Membranes were dehydrated by immersion, for 10 minutes each, in serial dilutions of ethyl alcohol: 50, 70, 80, 90, and 100%. They were then dried by incubation overnight at -80 °C in a closed box, coated by spatter of gold (SPI-Module; Structure Probe, Inc., West Chester, PA, USA), and finally examined by scanning electron microscope at Stony Brook University School of Engineering.
Collected data were examined for normality using frequency tables and histogram to check data distribution; the mean, median, and standard deviation were calculated using Shapiro-Wilk and Kolmogorov-Smirnov tests. Normally distributed data were analyzed by t-test and ANOVA followed by Tukey’s post hoc test, while nonparametric data were analyzed by Kruskal-Wallis test and then Mann–Whitney test with Bonferroni correction. The level of significance was set atp ≤ 0.05 using SPSS version 22 for Windows (IBM Corp., Armonk, NY, USA).
Table 1 demonstrates the mean and standard deviation of GMSCs colonies of the 3 experiments, with typical fibroblast morphology in P10 dishes after 14 days of in vitro culturing.
| Deviation type | Experiment 1 | Experiment 2 | Experiment 3 |
| SD | 8 | 5 | 3 |
| Mean | 17 | 11 | 22 |
Table 1: The mean and SD deviation of colony-forming unit experiments.
Table 2 demonstrates the GMSCs proliferation capacity of the 3 experiments, which showed high division properties, with close values in the 3 experiments.
| Experiment | Passage 1 | Passage 2 | Passage 3 | Total |
| Experiment 1 | 1.807 | 1.541 | 1.328 | 4.676 |
| Experiment 2 | 1.417 | 1.449 | 1.439 | 4.305 |
| Experiment 3 | 1.391 | 1.351 | 1.369 | 4.111 |
Table 2: Population doubling assay outcomes of the three experiments and the total assay outcome of each experiment.
Flow cytometric analysis was used to characterize cultured cells from gingival tissue at passage 5. Figure 1 shows the fluorescence intensity of expressed markers; CD105-Alexa 555 was 99%, CD73- FITC was 98.1%, CD90-APC was 99.9%, and CD 146-PE was 17.2%, which showed very high signal for the 3 main markers of mesenchymal stem cells, while the cells lacked the expression of the hematopoietic markers CD14, CD34, and CD45.

Figure 1: A-Graphs showing the intensity of fluorescence using flow cytometry of GMSC surface markers.
B-A representative image of colony-forming unit assay.
Staining the cells with Alizarin Red showed stained calcium deposits as orange-red aggregates. Alcian Blue staining of cells cultured in chondrogenic media showed chondrogenic glycoproteins in blue, and using Oil Red for staining cells cultured in adipogenic media showed red oil droplets within the cells. Using the same stain with similar cell lines cultured in regular culture media, no signs of cell differentiation deposits could be detected. Figure 1 shows representative image of cells cultured in differentiation media and their corresponding negative controls.
Table 3 shows the spectrophotometer reading of GMSCs at passage five versus those of plain media, where there was no significant difference between the scores of the three experiments.
| Experiment | GMSCs | Control | MTT |
| Experiment 1 | 2.604 | 0.951 | 1.653 |
| Experiment 2 | 2.345 | 0.943 | 1.402 |
| Experiment 3 | 2.404 | 1.291 | 1.113 |
Table 3: The spectrophotometer scoring of GMSCs MTT staining in comparison to plain media in the three experiments.
The 8-micron perforated polycarbonate membrane: Table 4 demonstrates the mean values of the total GMSCs migration to FBS chemo-attractant media versus plain media negative control, after 24-hour incubation, in the three experiments. Data showed nonparametric distribution, and the statistical analysis using Mann-Whitney test showed high significance statistical difference (p<0.001).
| 8-Micron polycarbonate | ||
| Experiment | FBS chemoattractant | Control |
| Experiment 1 | 21.8 | 0.6 |
| Experiment 2 | 21 | 0.2 |
| Experiment 3 | 19.4 | 1.8 |
Table 4: Values of mean migrating cell counts per 8 µm perforated membranes.
The 0.4 and 3-micron perforated collagen membranes: Table 5 and table 6 demonstrate the mean values of the total GMSCs migration to FBS as a chemoattractant versus plain media as a negative control after 24 hours of incubation, through 3 µm and 0.4 µm pore diameters, respectively, in the three experiments. Data showed nonparametric distribution, and the statistical analysis using Mann-Whitney test demonstrated statistically significant difference (p<0.05) as shown in figure 2, table 5 and 6.
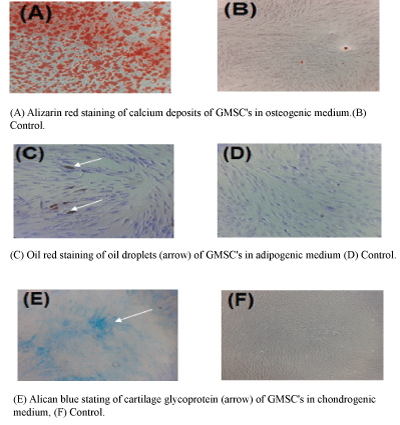
Figure 2: A-Alizarin Red staining of calcium deposits of GMSCs cultured in osteogenic induction media.
B-The same stain of GMSCs cultured in α-MEM and 10% FBS, showing no calcium deposits.
C-Oil Red staining of oil droplets within GMSCs cultured in adipogenic differentiation media.
D-The same stain of GMSCs cultured in α-MEM with 10% FBS, showing no oil droplets.
E-Alcian Blue staining of GMSCs cultured in chondrogenic induction media, showing blue stain of cartilage glycoprotein.
F-The same stain of GMSCs cultured in α-MEM with 10% FBS, showing no cartilage glycoprotein.
| 3-Micron collagen-coated PTFE | ||
| Experiment | FBS chemoattractant | Control |
| Experiment 1 | 5 | 0.4 |
| Experiment 2 | 7.4 | 0.6 |
| Experiment 3 | 3.8 | 1.8 |
Table 5: Values of mean migrating cell counts per 3 µm perforated membranes.
| 0.4-Micron collagen-coated PTFE | ||
| Experiment | FBS chemoattractant | Control |
| Experiment 1 | 1.8 | 0 |
| Experiment 2 | 1 | 0.2 |
| Experiment 3 | 1.8 | 0.2 |
Table 6: Values of mean migrating cell counts per 0.4 µm perforated membranes.
The 8 µm diameter perforated membrane group showed the highest cell migration values, followed by the 3 µm group, with the 0.4 µm group showing the least values. Data showed nonparametric distribution, and the Mann-Whitney test comparing the migrating cell counts of the three chemotaxis-experiment groups showed a statistical significant difference between the first and the second group and between the second and the third group, in addition to significant difference between the first group (8 µm pore diameter) and the third group (0.4 µm pore diameter) as shown in table 7 and figure 3.
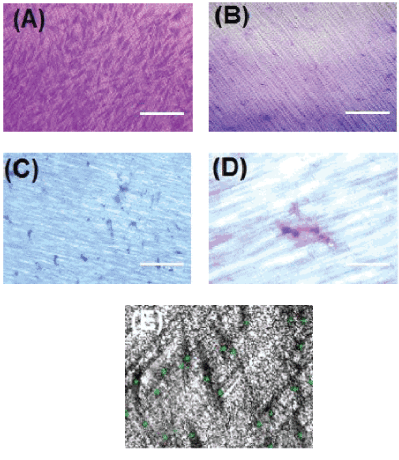
Figure 3: A-Crystal violet-stained GMSCs migrating through 3 µm diameter perforations of collagen membrane, FBS chemotaxis group, 10X, scale bar: 100 µm.
B-Crystal violet staining of perforated collagen membrane, negative control group, showing no cell migration.
C-Crystal violet-stained GMSCs migrating through 0.4 µm diameter perforated collagen membrane, FBS chemotaxis group, and 10X, scale bar: 100 µm.
D-Higher magnification of image C, showing single stem cell stained with crystal violet migrating through 0.4 µm pore diameter, 40X, scale bar: 20 µm.
E-Sample image of counting the cells migrating to the lower compartment of the 3 µm perforated collagen-coated membrane.
| Diameter | FBS chemoattractant | Control | Significance |
| Polycarbonate (8 µm) | 20.7** | 0.8 | p ≤ 0.001 |
| Collagen-coated PTFE (3 µm) | 5.3** | 0.93 | p ≤ 0.001 |
| Collagen-coated PTFE (0.4 µm) | 1.53* | 0.4 | p ≤ 0.05 |
Table 7: Comparison of mean migrating cell counts through 8, 3, and 0.4µm perforated membranes and their significance.
*High statistical significant difference.
*Statistical significant difference.
SEM analysis in all the experiments revealed polyhedral fibroblast morphology with long cytoplasmic extensions of GMSCs which migrated through the perforated membrane. In the 0.4 µm diameter group, perforated collagen-coated PTFE membranes showed a very limited number of migrating cells, where only the tips of the migrating cells could be seen in one of the images (Figure 4), which proves that the cells are actually going through the membrane and not through any other way.
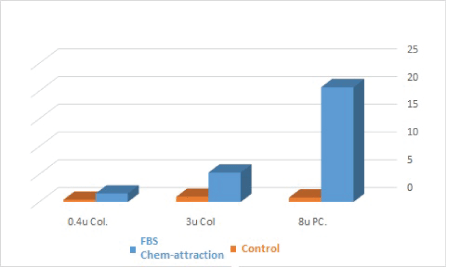
Figure 4: A bar chart comparing the migration of GMSCs through different microperforated membranes (blue: FBS chemotaxis group; orange: negative control group; 0.4u Col: 0.4 µm pore diameter, perforated collagen-coated membrane; 3u Col: 3 µm pore diameter, perforated collagencoated membrane; 8u PC: 8 µm pore diameter, perforated polycarbonic acid membrane).
The GMSCs that migrated through polycarbonate membrane seemed to look flatter in shape and spread over the membrane surface; on the contrary, cells that migrated through the collagencoated PTFE membrane looked more bulbous and were confined to the strands of the collagen. This indicates that the morphology of the GMSCs looked different over different materials that cells migrated through, figure 5.
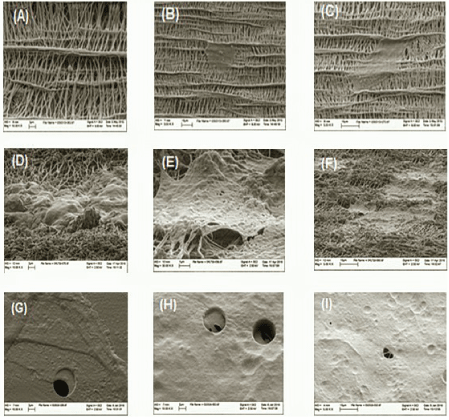
Figure 5: Images A to C show SEM of lower surface of 0.4 µm pore diameter of perforated collagen-coated membrane, GMSCs migrating through the membrane collagen strands, and FBS chemotaxis group. Images D to F show SEM of lower surface of 3 µm pore diameter of perforated collagen-coated membrane, GMSCs migrating through collagen strands, and FBS chemotaxis group. Images G to I show SEM of lower surface of 8-micron perforated poly-carbonate membrane, FBS chemotaxis group; we can note GMSCs migrating through the pore (arrows).
The critical-size periodontal bone defect suffers diminished regenerative power due to limited multipotent cells number and vascular supply; this causes its invasion by more proliferative tissue such as gingival epithelium or the highly vascular connective tissue [18]. GTR aims to prevent the migration of the gingival epithelium and connective tissue along the cementum wall of the pocket, creating a space for stabilization of the blood clot to allow the periodontal ligament cells with regenerative capacity to invade and achieve periodontal tissue regeneration [18].
The concept of perforated GTR membrane was first postulated by Reddi, et al. in 1987 [19], where demineralized bone allograft was supposed to, through its bone morphogenic proteins, stimulate the migration of undifferentiated mesenchymal cells by chemotaxis and increase their proliferation and differentiation into chondroblasts and then osteoblasts. It was suggested that the used occlusive barrier membranes in GTR might prevent the migration of the undifferentiated mesenchymal cells from the nearby tissues to the barrier-protected area, thereby reducing the bone-inducing effect of the demineralized bone matrix (DMB) [19].
In a study by Mardas, et al. it was concluded that the main reason for its result is the postulation that DBM would be chemo-attractant for mesenchymal stem cells, which lacked enough evidence, in addition to the huge pore sizes which would be nonselective for the adult stem cells; besides, the authors did not explain how the bone cells could invade the DBM graft through the occlusive capsule [6].
A recent study by Gamal, et al. suggested pore diameters of 0.2, 0.4, and 0.7 mm. They experimented with the migration of a mixed cell population of fibroblasts and GMSCs; using SEM, they reported Matrigel® Matrix (Cornig Scientific) extrusion through macro perforated membrane. There was no negative control in their study [20].
In the present study, a pilot in vitro experiment was conducted to test the migration of GMSCs through macro perforated (400 µm, 700 µm, and 1 mm pore diameters) transwell model, using FBS as a chemoattractant versus plain media as a negative control. The membranes lost their barrier effect, and all of the cells in the upper chamber sank to the floor of the lower chambers due to the effect of gravity after one hour of the experiment start point, and no cells attached to the collagen membranes were found in neither the chemotaxis groups nor the negative control, which would explain why the recent study by Gamal, et al. did not have a negative control group.
The SEM analysis showed GMSCs extruding through the pores of the membranes with different diameters. GMSCs had fibroblastlike morphology with cytoplasmic process extending inside the membranes pores in the chemoattractant groups; on the other hand, there was no sign of cell migration in the lower compartment of the negative control experiments, which proves that membranes were occlusive for cells in the absence of chemoattractant.
Comparing the morphology of cells over the two membrane materials used in this study, a difference in the morphological characteristics of the cells could be detected. On the polycarbonate membrane, GMSCs tend to be flatter in morphology showing more pseudopodia, some of which tend to be long and slender, while over the collagen membrane, the cells had rougher surface and tend to conform to the shape of collagen strands.
Within the limits of this study, we would conclude that micro perforated membranes of 0.4, 3, and 8 µm diameters are suitable for GMSCs migration, which could be used for cell homing technologies with suitable chemotaxis agents, without reducing the membranes’ barrier effect against undesired cells or affecting the membranes’ mechanical properties. More studies are required to test different chemotactic agents to attract certain cell lines in mixed cellular population.
Download Provisional PDF Here
Article Type: RESEARCH ARTICLE
Citation: Al Bahrawy M, Gamal A, Ghaffar KA, Iacono V (2018) In Vitro Migration Dynamics of Gingival Mesenchymal Stem Cells through Micro Perforated Membranes. Int J Dent Oral Health 4(4): dx.doi.org/10.16966/2378-7090.272
Copyright: © 2018 Al Bahrawy M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publication history:
All Sci Forschen Journals are Open Access